Calcium Chloride Hexahydrate for Phase-Change Heat Storage
I’ve been reading about some of the recent research into phase-change materials and what I’m reading about calcium chloride hexahydrate is very interesting. If you take calcium chloride — CaCl2 and mix it in roughly equal proportions with water it forms a structure where each calcium chloride molecule bonds to six water molecules. The resulting substance has a melting point of 29C or 84F. The heat of fusion is lower than that of ice, but it is denser, and it has about the same heat storage capacity per unit of volume as ice — but at a temperature close to room temperature. The melting point can be reduced by adding more water.
Calcium chloride is cheap, the components are among the most abundant elements on earth and commercially it is produced as a waste product of other industrial processes. It is essentially non-toxic.
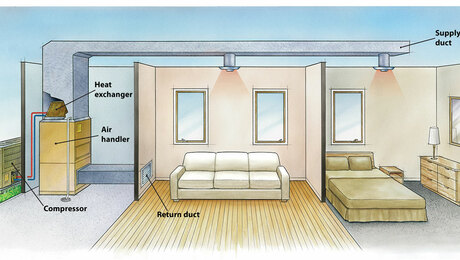
Conceptually it seems like seasonal storage of heat and “coolth” could be possible. A ton of cooling represents the cooling power of a ton of ice per 24 hours, or 12,000 BTU per hour. Imagine a house in a temperate climate where the heating load and cooling load are about the same and are 12,000 BTU/hr over the heating season. So you’d need the equivalent of one ton of ice per day. If you heating and cooling season were each 100 days, you’d need 100 tons of ice. A ton of ice is about a cubic yard, and calcium chloride hexahydrate has about the same energy density by volume, so you’d need about 100 cubic yards of storage. That could be a tank that was two yards deep, five yards wide and ten yards long. That’s a big tank, but something you could put in the basement of a new home.
So let’s say you have a big tank of 72-degree slush in your basement. You’d still need a heat pump, and in the winter you’d be pulling 95-degree heat out of the tank, and in the summer 50-degree cooling. It would still take some power, but I’d guess you’d be able to cut your power bill in half.
What if you don’t live some place where heating and cooling are balanced? If you’re in a heating-dominated climate, you need to dump excess heat into the tank. That you could do with thermal solar collectors that run all summer — you would have achieved the dream of thermal solar enthusiasts everywhere! If you’re in a cooling dominant climate it’s a little more complicated, sure you can use some for DHW but you’d probably end up having to dump heat outdoors in the winter.
The big problem with calcium chloride solutions is that they are prone to supercooling — they don’t always freeze at their freezing point. Pure solutions are particularly prone to supercooling, and introducing impurities is a way to prevent it. The current research is in finding impurities that cause the solution to reliably freeze, are cost-effective and otherwise practical.
GBA Detail Library
A collection of one thousand construction details organized by climate and house part








Replies
Hmm, no replies. I thought the nerds here would be all over this.
Let me add one more thought: This beats the pants off of geothermal.
A solution of about 45% calcium chloride and 55% water has a freezing point around 75F.
Seems better suited for larger scale users - it reminds me of Calmac's ice storage for skyscrapers. But that's daily cycling using ice, not annual cycling using some more expensive solution. I don't think the economics are there. It might outperform geothermal, but do we know what a 100 CY tub costs?
I figure the tub would be made as part of building the basement of a new home, it would basically be the cost of digging and pouring a deeper basement plus the cost of sealing it.
You'd need about 50 tons of CaCl2, I see it on Ali Baba for about $150/ton. So that would be about $7500.
So I don't see the cost immediately disqualifying the idea.
That pretty much disqualifies it. Ice does the same thing for $150 less/ton and is still meh economically. Cycling 1x per year kills the economics. An average heat load of 12,000btu/hr x 100 days x 24 hours/day is only 8440 kwh output, probably 2800 kwh input. Even if you zeroed the energy out, you’re only saving 2800 kwh/year. Probably an LCOE of $.20/kwh over twenty years on just the wholesale materials, maybe $1/kwh turnkey?
Damn, I think you're right. I should have run the numbers. Averaging 12K BTU/hr round the clock for 100 straight days seemed like a lot to me but it's really less than $50 worth of electricity. That's 28 million BTU and at 2800 kWh that's a SEER of 10.0 which isn't even that great.
DC,
It is interesting. How big a tank you you think you would need to incorporate to make this useful?
100 cubic yards for a typical residence. 6 feet deep, 15 feet wide, 30 feet long.
DC,
Our rainy climate means that the average house here can easily get its annual waters supply from the roof. What always becomes the problem is sufficient storage. Even dedicated advocates balk at the size of the tank necessary - partly because insurance companies are reluctant to cover houses with large interior water storage, so it has to be located in accessory structures. I suspect even absent the cost concerns others have raised, the stoage will be the deal breaker.
Another recent discussion on thermal solar reminded me of the houses designed by William Shurcliff. They had basements full or rock for storage, and attics covered by glass carboys of water. The houses worked, but the gymnastic involved didn't prove persuasive to many others. I wonder if this isn't unfortunately much the same?
A 2022 article: https://www.mdpi.com/1996-1073/15/3/824/pdf (addressing subcooling)
I also saw an article on corrosion, where various metals/alloys were tested.
Seems like most studies are coming out of China (including patent applications). The link uses a mix of glycerol and CCH:
"For example, the price of glycerol used in this paper is 36¥/500 g [$5.69/500 g]. However, the price of calcium chloride hexahydrate is 5.2¥/500 g [$0.82/500 g]."
"In this study, a novel CPCM based on CaCl2 6H2O was developed for air-conditioning
cold storage system. In the field of air-conditioning, the melting temperature of PCM should be in the range of 5–12 C [29]. To make the phase change temperature of CaCl2 6H2O appropriate for air-conditioning, glycerol was used as an additive to modify PCM. Due to the viscosity of glycerol, the synthesized CPCM has no phase separation and does not need to add thickener."
"After DSC test, it was found that when the mass ratio of calcium chloride hexahydrate to glycerol was 85:15, the phase change temperature of the composite phase change material was 11.8 C and the enthalpy of phase change was 112.86 J/g, which was suitable for the field of air-conditioning."
Enthalpy of 0.107 BTU/g or 107 BTU/kg (around $4 per kg?)
It will have to be an entirely closed loop system or the salt will suck all the moisture out of your house until the RH is about 20%. At a density of 1.7 g/cm3, if it costs $1.60/kg (per post #5), 100 yards will run you around $200,000. While not highly toxic in moderate quantities, it is quite caustic as well as corrosive to metal. Special considerations will be required for the tank material and wetted parts (plain steel or aluminum will not do) and for support of the tank and contents. Good luck!
I realize that's the standard answer when someone proposes thermal storage, I've made it myself. But I think this is different (like everyone proposing thermal storage!)
The tank would be just making the basement a few feet deeper. Insulate on sides and bottom with a few inches of foam, and put an EPDM bladder in. Then run joists over it and have a normal basement floor. It would actually be a very pleasant basement with a tank below that stays at 72F year-round. Heat exchanger would be just PEX pipe with water running into it. The whole thing would be sealed with no moving parts, no source of stress or strain.
I'd envision a water-to-water heat pump set so one end is at 50F and the other at 90F, with switchover valves so one end goes into the tank and the other end serves the house. It would use plain water as the medium and would require a tiny amount of refrigerant.
On Alibaba you can buy calcium chloride for $120/ton at 74% purity. If you needed 100 tons of 45% that would be about $7300.
Sorry, when I said "good luck", I wasn't trying to be facetious; just a lame attempt at humor. A small scale proof of concept sounds like a fun project. At 74%, I wonder what are the impurities, and how might they affect the heat capacity?
I'm pretty sure the only significant impurity in the 74% stuff is water. It bonds easily to water and forms hydrates with 1, 2, 4 or 6 water molecules. Dihydrate -- two water molecules -- is 26% water by weight.
Since the plan is to add water anyway there's no sense in paying to get it dehydrated.
There's no question that phase-change thermal storage works. It's been done and is being done all over, on special projects. As in all of these schemes, the devil is in the details. If the total system costs pencil out, it could be awesome. For most existing systems, the cost only pencils out in very specific sites and applications, and that mostly for daily storage, not seasonal. So far, you've estimated the cost of the phase change material itself. Go ahead and start adding in the cost of the tank and liner, maintenance, periodic replacement of the salt if/when it gets contaminated or over saturated, etc.
One of the challenges to seasonal phase change heat storage is whether or not you can guarantee that the reservoir holds ALL of the heat/cold needed for seasonal use. If not and a backup is required, then you're into the realm of having two complete (or nearly complete) HVAC systems and that boosts your system costs a lot. To guarantee that reservoir capacity is adequate, you'll have to design for worst-case seasons with a safety factor. I'd bet that the system size increases by a factor of 2 or more. Your big tank suddenly gets a whole lot bigger.
I'm not at all saying it won't work. It clearly could. I'm still a bit dubious that it can be done economically. Sure would be fun to design and build one though.
Might it make more sense to consider doing this as a storage mechanism to level out shorter periods of time? Is your fall/winter/spring solar potential sufficient that a couple of weeks of solar thermal storage would allow you to use it as your primary source of home heating? Just a thought to consider.
If you look at post #11 and #15, the economics just aren't there. The scale doesn't matter.
So I guess we are back to Martin's repeated assertion that "Solar thermal is really, really dead".
Yep. And I still maintain that CaCl2 beats the pants off of geothermal, so that would make it really, really, really dead.
I know I'm beating a dead horse here, but...
1. "If you take calcium chloride — CaCl2 and mix it in roughly equal proportions with water..."
Please don't try this at home, kids! The heat of solution of CaCl2 is about -83 kJoules/mole (heat is released when dissolved in water). Mixing 100 tons of CaCl2 with water will release 825,000 kJ of heat, enough to boil about 100 gallons of water.
2. You can't just toss a bunch of CaCl2 into water and hope to obtain a useful phase change material. The greatest energy density is in the 6-hydrated form, as stated. Water molecules are bound within the solid crystal lattice; this is not "free" water of solvation. Energy (196 BTU/kg) is required to melt from a crystalline solid to a liquid, at about 30C. Liquid CaCl2 6H2O is not the same as CaCl2 dissolved in water.
Deleted