Water – The Wonder and the Danger
We live on a watery planet. 70% of the earth’s surface is water (the same percentage of water in our bodies). It is the font and sustainer of life (SETI looks for water on other planets as the sine qua non of life). So why have our modern “green” building practices turned it into a monster? And how can we stop fighting it and turn it back into an ally?
GBA Detail Library
A collection of one thousand construction details organized by climate and house part
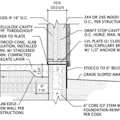
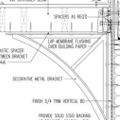






Replies
First, let's explore the magic and mystery of this most wonderful of all substances in the Universe.
Water is the only terrestrial substance that naturally exists in three distinct states: liquid, solid, vapor.
Water is essential for metabolism, photosynthesis and the thermoregulation of our bodies & the earth.
Solid water (ice) is less dense than liquid water and hence floats – this makes lakes freeze on top which allows aquatic life to survive the winter.
Water has an abnormally high melting point, which allows liquid water to cover the earth and create a breeding ground and habitat for life.
Water has the highest specific heat of all liquids except ammonia, which facilitates heat transfer in the atmosphere and oceans and moderates temperature extremes.
Water has exceptionally high surface tension, which allows drop formation and rain as well as capillary movement within plants and trees as tall as 450 feet.
Water absorbs infrared and ultraviolet light, which encourages photosynthesis and regulates atmospheric and oceanic temperatures.
Water is an excellent solvent for ionic salts and polar molecules (the universal solvent), which facilitates the transfer of nutrients in metabolism and in hydrologic cycles.
Water has the highest heat of vaporization of any molecular liquid.
Water has the highest thermal conductivity of any liquid (except for liquid metals)
Hot water freezes faster than cold water.
Boiling water will turn instantly to snow at temperatures below -25°.
Water has at least 5 liquid phases and 14 solid phases (remember Ice 9?)
When supercooled, water will turn instantly to ice when disturbed.
When superheated, water will turn instantly to vapor when disturbed.
Water at -184° F turns super-viscous like molasses.
Water at -211° F turns amorphous like glass.
Homeopathy is correct that water has a "memory" of what was once dissolved in it and retains the "essence".
There is evidence that water also retains thoughts, moods & conscious intentions (see Dr. Emoto's research).
How do buildings get wet, what happens when they do, and how do they dry out before getting into trouble?
First, it's essential to note that, as building scientist Anton TenWolde states, "You need to assume that the building will get wet, somehow, at some point in time. Stuff happens."
We all know that buildings often get wet from exterior leakage: through poorly-detailed flashings or failed caulk joints, or by wind-driven rain, or solar vapor drive, or ice dams on unventilated roofs. Mitigating those problems is largely a matter of good design, good craft, and good maintenance.
We also know that building envelopes get wet from the inside: from vapor diffusion and air movement – mechanisms of mass transfer that are facilitated by modes of energy flux. We also now know that movement of warm moist air is an order of magnitude greater a problem than vapor diffusion because of the time scale: air moves quickly, vapor diffuses slowly. But, the more air-tight we make our homes (and any home built to today's energy codes is relatively tight) the more we need to consider the long-term (seasonal) ramifications of vapor diffusion and consequent condensation and accumulation (more on this later).
But no evaluation of moisture dynamics in a building is complete without consideration of short- and long-term moisture storage, redistribution and drying potential in building materials.
As TenWolde explains: "A moisture problem occurs when wetting exceeds drying over a long period of time. But it is important to know several things: How wet does it get? How long does it stay wet? And what is the temperature while it is wet? Because if it is cold enough the mold won’t grow well, and decay organisms won’t do well. How does this information translate into the design of a building? …So you need a moisture-tolerant design."
Martin's recent blogs ("Calculating the Minimum Thickness of Rigid Foam Sheathing" and "How To Avoid Condensation in Your Walls") have attempted to clarify the notion of condensation, though I think they may have added to the confusion.
Martin has begun to differentiate between "condensation" (which he now puts in quotes) and moisture accumulation. While it's true that moisture accumulation is the problem, absent bulk water leakage it occurs either by prolonged high relative humidity or because of condensation of water vapor that is transported either by air movement through openings or by diffusion through building materials. So condensation is an important concept that must be properly understood.
Martin: "Condensation occurs when water vapor condenses on a cold, hard surface like glass or an aluminum beer can. Condensation generally does not occur on a hygroscopic material like wood framing, plywood sheathing, or cellulose (however, frost can occur on these materials)."
This confuses two uses of the term "condensation": the simple change of state of water vapor to liquid water (scientific), and the visible droplets of water that we see on a cold window (vernacular). The first is a dynamic process, the second is the visible result. The confusion should be evident in his parenthetical qualification – frost forms when water vapor freezes as it condenses; or, in other words, the dew point surface is below freezing. Since there cannot be frost without condensation (except in extreme cold), condensation occurs even within fibrous insulation.
It's clear where Martin's confusion comes from. He quotes two respected building scientists who are struggling to clarify this common misunderstanding.
William Rose: "Condensation is the change in phase from vapor to liquid water. Condensation occurs typically on materials such as glass or metal that are not porous or hygroscopic and on capillary porous materials that are capillary saturated. Use of the term “condensation” to refer to change in phase between vapor and bound water in capillary or open porous materials is discouraged."
Rose's first sentence is the scientific definition. His second sentence, however, uses the term "condensation" more in the vernacular sense as visible or tangible condensation and discourages the use of the term as applied to hygroscopic or porous materials, even though it's scientifically correct, because he believes it leads to erroneous conclusions for lack of a more complete understanding of hygro-thermal dynamics.
Anton TenWolde: "We thought there would be a problem with condensation in the insulation, but all the action happens on the sheathing and the interior vapor barrier. We’ve confirmed this by opening up walls. The action is never in the insulation."
What TenWolde is saying is that the "action" or the accumulation and consequent moisture problems happen at the interfaces of materials where the moisture goes "bump" against a relatively impermeable substance.
The reason for the confusion and the attempts at clarification is because of the complexity of the more subtle moisture dynamics within relatively "open" materials. These dynamics are very difficult to model.
For instance, there are as many as five difference hygric phases or regimes within a porous hygroscopic material and it's quite possible for moisture to move in two opposite directions simultaneously within the same material. Water vapor, which is driven by vapor pressure differential, can move from inside to out while liquid water can move by surface diffusion, driven by relative humidity differential and solar drive, from outside to in. Until the capillaries are saturated with liquid water, water vapor can move within the open pores wherever it's driven, even against the flow or the water that lines the pores. After saturation, it requires the forces of kinetics (wind) and gravity to move the bulk water.
In fact, in relatively dry material conditions, vapor diffusion dominates. As materials get moist, surface diffusion of liquid (adsorption) begins to take over. And in wet conditions, capillarity and bulk flow dominates (as the pores are too full of water to allow vapor to move).
Similarly, the opposing mechanisms of evaporation and condensation are happening everywhere all the time, as well as ab/adsorption and desorption. So, to say that condensation is not occurring anywhere that water vapor can get to is simply incorrect. But, if evaporation and condensation are in balance, then there is no accumulation. The accumulation, and hence the problems, occur when the rate of condensation exceeds the rate of evaporation and the accumulation is more than the material can safely store.
It's important to note that, even in a steady-state environment, the moisture content of hygroscopic materials can rise to the danger point. This happens when there is a long period of high relative humidity, which gradually increases the equilibrium moisture content of the sorbent materials. Constant high humidity, as we all know, is a dangerous thing (except to fungi, mold and decay organisms).
So vapor pressure and temperature gradients drive vapor diffusion - while concentration gradients, relative humidity differentials and surface tension drive liquid diffusion and capillary conduction.
While those pressures and gradients determine the rate of moisture migration, it’s the hygroscopicity of materials that determines the rate and quantity of moisture storage. Hygroscopicity is a function of the vapor diffusivity of a material (rate of internal water vapor diffusion), liquid adsorptivity (rate of liquid uptake) and liquid storage capacity (porosity & absorptivity). However, for a hygroscopic material to function well as a diurnal (daily) moisture buffer, it has to have high diffusivity and moisture storage capacity (density and porosity), but only moderate absorptivity. If it absorbs water too quickly becomes saturated, then the pores will be closed to deeper penetration of water vapor. And diurnal storage and release is dependent on relatively low air exchange rates to allow sufficient contact time for sorption and release. Interestingly, air change rates of 0.25 ACH or less is ideal for moisture buffering and also for indoor air quality and energy efficiency.
The materials that perform best for diurnal moisture control are the ones least used in today's homes, such as endgrain wood and earthen plasters. After those, but considerably lower on the scale, are the traditional materials such as plaster, wood, brick and lime mortar.
As the new IRC code on the use of class III vapor retarders (vapor semi-permeable, like ordinary latex paint) indicates, there is more than one acceptable strategy for keeping a building's sheathing from rotting. The increasingly popular one is to use exterior rigid foam to keep the sheathing (most of the winter) above the dew point. But the other method recognized in the same IRC table (N1102.5 in the 2006 version and R601.3.1 in the 2009 edition) is a vapor permeable sheathing with a ventilated cladding for ease of drying to the exterior. Though the IRC doesn't include it in the chart, wooden board sheathing is the most durable and vapor open of all and, with vapor open cladding like clapboards, does not (in my estimation) require a ventilated gap except perhaps in very high rain and wind zones.
I stumbled upon a pretty fine book recently
Water by Leopold Luna
The Bill Rose" Water Book" is very good too except it is $$ and not so easy to read
The Leopold Luna Book is cheap, Not-So-Thick
and Not-So-Hard-To-Read
And...... It has Pictures
Robert,
I would like to hear your thoughts about this
I am smitten with water
Wow,
you are a blazing typist!
you posted 4 pages while I was posting my comment.
John,
I think you mean Luna Leopold, the son of the late great Aldo Leopold, author of A Sand County Almanac (1949), with it's powerful chapter titled Thinking Like a Mountain (which I borrowed for the title of my last and best class at Yestermorrow).
Aldo Leopold, from A Sand County Almanac:
"That land is a community is the basic concept of ecology, but that land is to be loved and respected is an extension of ethics."
"A land ethic, then, reflects the existence of an ecological conscience, and this in turn reflects a conviction of individual responsibility for the health of the land. Health is the capacity of the land for self-renewal. Conservation is our effort to understand and preserve this capacity. A thing is right when it tends to preserve the integrity, stability and beauty of the biotic community. It is wrong when it tends otherwise."
"Examine each question in terms of what is ethically and aesthetically right, as well as what is economically expedient."
And...what was it you wanted me to comment on? That you are "smitten with water"?
When I'm channeling the Wisdom of the Universe, I can't even see my fingers moving on the keypad.
Actually, all the pages were already typed. I just posted them in installments - kind of like Burma Shave signs.
I proposed / To Ida / Ida refused / Ida won my Ida / If Ida used / Burma-Shave
Keep well / To the right / Of the oncoming car / Get your close shaves / From the half pound jar / Burma-Shave
Past / Schoolhouses / Take it slow / Let the little / Shavers grow / Burma-Shave
Hardly a driver / Is now alive / Who passed / On hills / At 75 / Burma-Shave
If you dislike / Big traffic fines / Slow down / Till you / Can read these signs / Burma-Shave
It's best for / One who hits / The bottle / To let another / Use the throttle / Burma-Shave
Train approaching / Whistle squealing / Stop / Avoid that run-down feeling / Burma-Shave
Don't take a curve / At 60 per / We hate to lose / A customer / Burma-Shave
You can drive / a mile a minute / but there's no / future in it / Burma-Shave
If you / Don't know / Whose signs / These are / You can't have / Driven very far
Explain hot water freezes faster than cold water.
History of the Mpemba Effect
The fact that hot water freezes faster than cold has been known for many centuries. The earliest reference to this phenomenon dates back to Aristotle in 300 B.C. The phenomenon was later discussed in the medieval era, as European physicists struggled to come up with a theory of heat. But by the 20th century the phenomenon was only known as common folklore, until it was reintroduced to the scientific community in 1969 by Erasto Mpemba, a Tanzanian high school student. Since then, numerous experiments have confirmed the existence of the "Mpemba effect"
The earliest known reference to this phenomenon is by Aristotle, who wrote:
"The fact that water has previously been warmed contributes to its freezing quickly; for so it cools sooner. Hence many people, when they want to cool hot water quickly, begin by putting it in the sun. . ."
Around 1461, the physicist Giovanni Marliani, in a debate over how objects cooled, said that he had confirmed that hot water froze faster than cold. He said that he had taken four ounces of boiling water, and four ounces of non-heated water, placed them outside in similar containers on a cold winter day, and observed that the boiled water froze first. Marliani was, however, unable to explain this occurrence.
Later, in the 1600's, it was apparently common knowledge that hot water would freeze faster than cold. In 1620 Bacon wrote "Water slightly warm is more easily frozen than quite cold", while a little later Descartes claimed "Experience shows that water that has been kept for a long time on the fire freezes sooner than other water".
In time, a modern theory of heat was developed, and the earlier observations of Aristotle, Marliani, and others were forgotten, perhaps because they seemed so contradictory to modern concepts of heat. However, it was still known as folklore among many non-scientists in Canada, England, the food processing industry, and elsewhere.
It was not reintroduced to the scientific community until 1969, 500 years after Marliani's experiment, and more than two millennia after Aristotle's "Meteorologica I". The story of its rediscovery by a Tanzanian high school student named Mpemba is written up in the New Scientist. The story provides a dramatic parable cautioning scientists and teachers against dismissing the observations of non-scientists and against making quick judgements about what is impossible.
In 1963, Mpemba was making ice cream at school, which he did by mixing boiling milk with sugar. He was supposed to wait for the milk to cool before placing it the refrigerator, but in a rush to get scarce refrigerator space, put his milk in without cooling it. To his surprise, he found that his hot milk froze into ice cream before that of other students. He asked his physics teacher for an explanation, but was told that he must have been confused, since his observation was impossible.
Mpemba believed his teacher at the time. But later that year he met a friend of his who made and sold ice cream in Tanga town. His friend told Mpemba that when making ice cream, he put the hot liquids in the refrigerator to make them freeze faster. Mpemba found that other ice cream sellers in Tanga had the same practice.
Later, when in high school, Mpemba learned Newton's law of cooling, that describes how hot bodies are supposed to cool (under certain simplifying assumptions). Mpemba asked his teacher why hot milk froze before cold milk when he put them in the freezer. The teacher answered that Mpemba must have been confused. When Mpemba kept arguing, the teacher said "All I can say is that is Mpemba's physics and not the universal physics" and from then on, the teacher and the class would criticize Mpemba's mistakes in mathematics and physics by saying "That is Mpemba's mathematics" or "That is Mpemba's physics." But when Mpemba later tried the experiment with hot and cold water in the biology laboratory of his school, he again found that the hot water froze sooner.
Earlier, Dr Osborne, a professor of physics, had visited Mpemba's high school. Mpemba had asked him to explain why hot water would freeze before cold water. Dr Osborne said that he could not think of any explanation, but would try the experiment later. When back in his laboratory, he asked a young technician to test Mpemba's claim. The technician later reported that the hot water froze first, and said "But we'll keep on repeating the experiment until we get the right result." However, repeated tests gave the same result, and in 1969 Mpemba and Osborne wrote up their results.
In the same year, in one of the coincidences so common in science, Dr Kell independently wrote a paper on hot water freezing sooner than cold water. Kell showed that if one assumed that the water cooled primarily by evaporation, and maintained a uniform temperature, the hot water would lose enough mass to freeze first. Kell thus argued that the phenomenon (then a common urban legend in Canada) was real and could be explained by evaporation. However, he was unaware of Osborne's experiments, which had measured the mass lost to evaporation and found it insufficient to explain the effect. Subsequent experiments were done with water in a closed container, eliminating the effects of evaporation, and still found that the hot water froze first.
Subsequent discussion of the effect has been inconclusive. While quite a few experiments have replicated the effect, there has been no consensus on what causes the Mpemba effect.
However, I'll explain the cause in the next posting. It's really quite simple.
Why Does Hot Water Freeze Faster than Cold Water
Liquid water exists in at least three distinct states:
1) when it's quite hot, the molecules have enough kinetic energy to dance alone
2) at mid-temperatures, the molecules tend to cluster together into icosohedrals
3) at cold temperatures, the clusters clump together in a very slow motion dance
In order for water to freeze, four conditions are necessary:
1) it must be at or below 32°F
2) it must separate into individual molecules (overcome the strong hydrogen bonds)
3) find new hydrogen bonding partner molecules
4) and re-arrange into hexagonal structure (ice crystals)
When cold, sluggish, clumped water molecules are cooled to freezing, it takes them forever to break away from their dance partners and rearrange into symmetrical hexagonal prisms – in fact, they often have to fall well below freezing before they get around to it. Moderate temperature water molecules can dissociate more quickly to find new dancing partners, but they're still a bit slow. Very hot water molecules, already solo dancing and full of energy, can immediately collect a set of dance partners for the crystal dance when they're quickly cooled to the freezing point.
And that's why most Zambonis use 140° to 160° water to resurface skating rinks.
Robert,
Thank you for a wonderful tutorial on the pleasures and perils of water. And also for the first lucid explanation of the Mpemba Effect I've ever seen. Concerning building details would you, in general, consider the Pacific Northwest as sufficiently wet and windy as to justify the use of a rainscreen siding design?
YES, sorry...... Luna B. Leopold...the title is simply "WATER"
1966 Time Inc
Available used from around $6
After reading Leopold's book .....I think I can go back and better understand Bill Rose's book
(If my good friend would only return it)hint,hint
It is impossible to buy Bill's Book Used .. no one wants to give it up
And if you get a copy DO NOT LOAN IT OUT ;---)
I should have said I am looking forward to your (Robert's)comments.
Your comments came blazing thru before I could post
Your comments were worth the wait ;--)
Hello Robert,
I cannot comment on all you wrote here. There’s quite a bit, as another commenter noted. The summary you gave of what I wrote on condensation is accurate. Common use of the term does drift away from the scientific use. One can argue that ad/absorbed water is in fact condensed—it is—but the energies involved in binding water to sites in wood and brick vary widely, while the energy in forming droplets of pure water is singular.
“Condensation” on windows is (sort of) binary, while wetness in porous and hygroscopic materials forms a continuum from very dry to saturated and beyond. The term “condensation” does a poor job of capturing what happens in opaque building systems, so I recommend dropping it from such use. ASHRAE 160 does a pretty good job of applying pass/fail criteria to assemblies with moisture storage. I suspect that the confusion arises because people want no more than a binary understanding about the condition of their building assemblies—ok or not ok. The use of the term “condensation” regarding buildings occurred more than 100 years ago in the architecture literature, while its first mention in the engineering literature is from the mid 1930s, with the introduction of insulation. The architects wanted to know only if it was going to be ok. “Condensation” is a convenient framework for making an analog continuum look binary.
Your comment #1 is an interesting and edifying summary of what water is and does. I'm skeptical of the more mystical properties you assign to water such as memory. Open—but skeptical.
I once wanted to test the theory that hot water freezes faster than cold water, being skeptical. I took two identical glass containers, filled one with hot tap water and one with cold, and stuck thermocouples in both, and placed them side-by-side in the freezing compartment of the 60 year-old Hotpoint refrigerator in our break area. Imagine my surprise when, yes, the hot water took a giant nosedive into colder temperatures and froze much more quickly than the cold water did. So I opened the fridge and took out the cold-water container, then the hot-water container, but it didn't budge. While the cold-water container had rested on a thin layer of frost in the freezer, the hot-water container had melted the frost, and formed a strong thermal bridge to the chilled aluminum enclosure. Oops, bad experiment. Gotta watch those boundary conditions. I tried it again, this time resting both containers on a scrap of polystyrene. This time the results seemed to indicate that the rate of temperature loss, assuming all boundary conditions are managed, is the same for hot and cold water. The curve from 60F to freezing was the same for both samples, even though one had started up at 130F. It undercut the “memory” theory.
I must stop now, and probably will not be able to get back to this blog any time soon, and won’t be able to provide followup. Good luck with the discussion.
Bill Rose
Bill Rose,
It is all your fault.
It was your book that started my quest to TRY and better understand water.
I have a long way to go.... but I am trying
So... You are going to tease us and then run away? :-0
Thanks Robert for posting.
Robert,
Thanks for your explanations and introduction of this topic. I agree with you that board sheathing is robust, and I have long advocated board sheathing. Most of the homes I have built included board sheathing.
I certainly strive to avoid confusion, and I don't agree with you that my blogs on this topic are evidence of confusion. I agree with Bill Rose that "The term 'condensation' does a poor job of capturing what happens in opaque building systems, so I recommend dropping it from such use."
Lately, I have been striving to avoid the use of the term "condensation" when discussion moisture accumulation in sheathing; occasionally, however, I have been overruled by other editors at GBA. (That's how the word "condensation" ended up in the title of one of my recent blogs.)
All discussions of this topic, including your recent posts, are welcome, as we all strive to banish confusion and educate each other on building science topics.
Bill Rose said: "I tried it again, this time resting both containers on a scrap of polystyrene. This time the results seemed to indicate that the rate of temperature loss, assuming all boundary conditions are managed, is the same for hot and cold water. The curve from 60F to freezing was the same for both samples, even though one had started up at 130F. It undercut the “memory” theory."
Do I understand Bill to say that there is no Mpemba effect, only the surprising results from a poorly designed experiment?
John,
I think what Bill Rose was saying is that his own experiments were poorly designed and controlled for the multitude of variables. What Bill was apparently measuring was only the rate of heat loss, not the rate or timing of freezing, which is the core of the Mpemba effect.
There have been many laboratory replications of the Mpemba effect, though little consensus about the cause.
Here is a link to a graph of the heat loss and freezing times for hot and cold water. The slopes are identical (heat loss rate), but because the hot water starts farther upslope it takes longer to get to freezing temperatures. However, once there, the hot water starts freezing sooner, requiring less supercooling, but takes longer to completely freeze.
http://www.btinternet.com/~martin.chaplin/images/mpemba.gif
Here are some more anomalies of water from an excellent (and very technical) website about water.
Anomalous properties of water
http://www.lsbu.ac.uk/water/anmlies.html
The anomalous properties of water are those where the behavior of liquid water is quite different from what is found with other liquids. Frozen water (ice) also shows anomalies when compared with other solids. Although it is an apparently simple molecule (H2O), it has a highly complex and anomalous character due to its intra-molecular hydrogen bonding. As a gas, water is one of lightest known, as a liquid it is much denser than expected and as a solid it is much lighter than expected when compared with its liquid form. An interesting history of the study of the anomalies of water has been published.
As liquid water is so common-place in our everyday lives, it is often regarded as a ‘typical’ liquid. In reality, water is most atypical as a liquid, behaving as a quite different material at low temperatures to that when it is hot. It has often been stated that life depends on these anomalous properties of water. In particular, the high cohesion between molecules gives it a high freezing and melting point, such that us and our planet is bathed in liquid water. The large heat capacity, high thermal conductivity and high water content in organisms contribute to thermal regulation and prevent local temperature fluctuations, thus allowing us to more easily control our body temperature. The high latent heat of evaporation gives resistance to dehydration and considerable evaporative cooling. Water is an excellent solvent due to its polarity, high dielectric constant and small size, particularly for polar and ionic compounds and salts. It has unique hydration properties towards biological macromolecules (particularly proteins and nucleic acids) that determine their three-dimensional structures, and hence their functions, in solution. This hydration forms gels that can reversibly undergo the gel-sol phase transitions that underlie many cellular mechanisms. Water ionizes and allows easy proton exchange between molecules, so contributing to the richness of the ionic interactions in biology.
At 4°C (39°F) water expands on heating or cooling. This density maximum together with the low ice density results in (i) the necessity that all of a body of fresh water (not just its surface) is close to 4°C (39°F) before any freezing can occur, (ii) the freezing of rivers, lakes and oceans is from the top down, so permitting survival of the bottom ecology, insulating the water from further freezing, reflecting back sunlight into space and allowing rapid thawing, and (iii) density driven thermal convection causing seasonal mixing in deeper temperate waters carrying life-providing oxygen into the depths. The large heat capacity of the oceans and seas allows them to act as heat reservoirs such that sea temperatures vary only a third as much as land temperatures and so moderate our climate (for example, the Gulf stream carries tropical warmth to northwestern Europe). The compressibility of water reduces the sea level by about 40 m giving us 5% more land. Water's high surface tension plus its expansion on freezing encourages the erosion of rocks to give soil for our agriculture.
Notable amongst the anomalies of water are the opposite properties of hot and cold water, with the anomalous behavior more accentuated at low temperatures where the properties of supercooled water often diverge from those of hexagonal ice. As (supercooled) cold liquid water is heated it shrinks, it becomes less easy to compress, its refractive index increases, the speed of sound within it increases, gases become less soluble and it is easier to heat and conducts heat better. In contrast as hot liquid water is heated it expands, it becomes easier to compress, its refractive index reduces, the speed of sound within it decreases, gases become more soluble and it is harder to heat and a poorer conductor of heat. With increasing pressure, cold water molecules move faster but hot water molecules move slower. Hot water freezes faster than cold water and ice melts when compressed except at high pressures when liquid water freezes when compressed. No other material is commonly found as solid, liquid and gas.
And below, from the same author, is a discussion of the vital importance of the properties of water for the existence and continuation and evolution of life, including a discussion of the threat to life posed by a rather small deviation in the existing strength of those magical Hydrogen bonds.
That water is not only so mysterious and anomalous in its properties, but also so perfectly poised between possible variations to allow life to exist in the Universe is perhaps the strongest scientific indication of the Intelligence of the Universe (or God, if you'd like).
Water Structure and Science
by Martin Chaplin
http://www.btinternet.com/~martin.chaplin/sitemap.html
Hydrogen bonding in water
Hydrogen bonding forms in liquid water as the hydrogen atoms of one water molecule are attracted towards the oxygen atom of a neighboring water molecule.
In a water molecule (H2O), the oxygen nucleus with +8 charges attracts electrons better than the hydrogen nucleus with its +1 charge. Hence, the oxygen atom is partially negatively charged and the hydrogen atom is partially positively charged. The hydrogen atoms are not only covalently attached to their oxygen atoms but also attracted towards other nearby oxygen atoms. This attraction is the basis of the 'hydrogen' bonds.
Can life exist without water?
Water and life are closely linked. This has been recognized throughout history by civilizations and religions and is still the case with scientists today. Liquid water is required for life to start and for life to continue. No enzymes work in the absence of water molecules. No other liquid can replace water. We are very fortunate, therefore, that our planet is so well endowed. Water is a common material in the Universe, being found as widely dispersed gaseous molecules and as amorphous ice in tiny grains and much larger asteroids, comets and planets, but water needs particularly precise conditions to exist as a liquid as it does on Earth. It is most likely that this water arrived from multiple sources, such as comets and asteroids, somewhat after solid planet Earth was formed.
Water possesses particular properties that cannot be found in other materials and that are required for life-giving processes. These properties are brought about by the hydrogen-bonded environment particularly evident in liquid water. If aqueous hydrogen bonds were actually somewhat stronger, then water would behave similar to a glass, whereas if they were weaker then water would be a gas and only exist as a liquid at sub-zero temperatures.
It is found that if the hydrogen bond strength was slightly different from its natural value then there may be considerable consequences for life. Water would not be liquid on the surface of Earth at its average temperature if the hydrogen bonds were as little changed as 7% stronger or 29% weaker. The temperature of maximum density naturally occurring at about 4°C (39°F) would disappear if the hydrogen bonds were just 2% weaker. Major consequences for life are found if the hydrogen bonds did not have their natural strength. Even very slight strengthening of the hydrogen bonds may have substantial effects on normal metabolism. Water ionization becomes much less evident if the hydrogen bonds are just a few percent stronger, but pure water contains considerably more H+ ions if they are few percent weaker. The important alkali metal ions Na+ and K+ lose their distinctive properties if the hydrogen bonds are 11% stronger or 11% weaker respectively. Hydration of proteins and nucleic acids depends importantly on the relative strength of the biomolecule-water interactions as compared with the water-water hydrogen bond interactions. Stronger water hydrogen bonding leads to water molecules clustering together and so not being available for biomolecular hydration. Generally, the extended denatured forms of proteins become more soluble in water if the hydrogen bonds become substantially stronger or weaker. If the changes in this bonding are sufficient, present natural globular proteins cannot exist in liquid water.
Consequences of changes in water’s hydrogen bond strength
No Hydrogen-bonding at all - No life
Hydrogen bonds slightly weaker - Life at lower temperatures
No change - Life as we know it
Hydrogen bonds slightly stronger - Life at higher temperatures
Hydrogen bonds very strong - No life
Intriguingly, liquid water acts in subtly different manners as circumstances change, responding to variations in the physical and molecular environments and occasionally acting as though it were present as more than one liquid phase. Sometimes liquid water is free flowing whilst at other times, in other places or under subtly different conditions, it acts more like a weak gel. Shifts in the hydrogen bond strength may fix water’s properties at one of these extremes to the detriment of processes requiring the opposite character. Evolution has used the present natural responsiveness and variety in the liquid water properties such that it is now required for life as we know it. DNA would not form helices able to both zip and unzip without the present hydrogen bond strength. Enzymes would not possess their 3-D structure without it, nor would they retain their controlled flexibility required for their biological action. Compartmentalization of life’s processes by the use of membranes with subtle permeabilities would not be possible without water’s intermediate hydrogen bond strength.
Quite small percentage changes in the strength of the aqueous hydrogen bond may give rise to large percentage changes in such physical properties as melting point, boiling point, density and viscosity. Some of these potential changes may not significantly impinge on life’s processes, (e.g. compressibility or the speed of sound) but others are of paramount importance.
Strengthening hydrogen bonding has particularly important effects on viscosity and diffusion.
Effect of water hydrogen bond strength on melting and boiling point
Bond strength increases affect the melting point and how bond strength decreases affect the boiling point. The resulting relationship shows that water would freeze at the average surface temperature of Earth (15°C / 59°F) with a 7% strengthening in water’s hydrogen bond or it would boil on a 29% weakening. At our body temperature (37°C / 98.6°F) the strengthening required for freezing is 18% and the weakening required to turn water into steam is 22%.
Conclusions
It is apparent that small changes of a few percent would not be threatening to life in general but changes in excess of 10% may cause a significant threat. The overall conclusion to be drawn is that water’s hydrogen bond strength is poised centrally within a narrow window of its suitability for life.
Robert,
I took the Bill Rose comments the same way as John H.
hmmm.
"Homeopathy is correct that water has a "memory" of what was once dissolved in it and retains the "essence".
There is evidence that water also retains thoughts, moods & conscious intentions."
I'm disappointed that your enumeration of the Magical qualities of water didn't include any empirical evidence for the above characteristics.
John,
A list is a list, not an explanation.
I did, however, point you to the man who has done extensive research into water's "higher" qualities, and the extremely dilute solutions of homeopathy and flower essence medicine has been studied for many years. Perhaps you don't want to bother doing your own research.
From the same scientific source as the recent posts:
http://www.btinternet.com/~martin.chaplin/memory.html
Liquid water is clearly a very complex system even before the further complexity of molecular clusters, gas-liquid and solid-liquid surfaces, reactions between these materials, the consequences of physical and electromagnetic processing and the addition of ethanol are considered. Any or a combination of these factors may cause 'memory' of past solutes and processing in water. Some of these solutions are capable of causing non-specific clinical effects whereas others may cause effects specifically linked to the solution's history.
Other interesting examples of the memory of water are the Mpemba effect and the observation that hot water pipes are more likely to burst than adjacent cold water pipes. In both effects, water seems to remember whether it has been recently hot or cold even when subsequently cooled. The Mpemba effect is a well proven phenomenon that also seems to be caused by unexpected solute and time effects.
By the way, I didn't miss this question, I ignored it - since I am not inclined to have dialog with nameless people.
If you'd share your full name, I'd be glad to answer.
Well? Are you asking US?
Or do you have a solution?
Drink at least four glasses of water every day (must be in a glass, as this effects the ortho-molecular structure and the memory imprinting of the water).
Oh, perhaps you mean in terms of building technology?
As I've stated many times in this forum, I believe that a deep understanding of hygro-thermal principles and mechanics (hardly seem like sufficient terms to describe the mysteries of water and energy, which science doesn't yet fully understand) would lead one to the conclusion that it is as foolish to try to "control" moisture as it is for the US Army Corps of Engineers to try to control the Mississippi River or the bayous of Louisiana.
Hence, it's an exercise in futility (or hubris, or both) to attempt to design and build the "perfect wall", if by this one means a building envelope that can resist all the potential incursions and impacts and consequences of moisture.
The "solution" to living with the Mississippi River or the Gulf Coast weather is not to dam it up and limit and control its flow, but rather to adjust our lives and our built environment to coexist with forces of nature that are much greater than civilization (whatever that is*).
The "solution" to creating livable and durable shelters is not to try to dam up the water and energy flows but to use methods and materials that have evolved with nature's fluid environment and have an innate intelligence that is greater than our own.
[* endnote: When a journalist asked Gandhi what he thought of Western civilization, he responded "I think it would be a good idea".]
Robert,
I don't agree that "Other interesting examples of the memory of water are the Mpemba effect and the observation that hot water pipes are more likely to burst than adjacent cold water pipes."
This has nothing to do with the Mpemba Effect, which most scientists, including Bill Rose, do not give any credit to.
The reason that hot water pipes freeze first is the existence of a toilet in most domestic plumbing systems. Toilet tank valves provide a convenient way for cold-water pipes to relieve the pressure of an expanding ice plug.
Hot water pipes have a relief valve at one end of the system (the PT relief valve on the hot-water tank), but not on the far end of the ice plug -- and so the hot water pipe is the first to burst.
Martin,
The quote you disagree with is not mine, but that of one of the word's leading authorities on water, Martin Chaplin, BSc, PhD, Chartered Chemist, Fellow of the Royal Society of Chemistry, Emeritus Professor of Applied Science at London South Bank University.
The Mpemba effect has been replicated by many scientists, though not with the consistency that would allow for general acceptance (though this is undoubtedly related to poorly controlled variables, such as in Bill Rose's quick and dirty refrigerator trials). I think most scientists, contrary to your claim, accept that this occurs but cannot agree on a cause - and that is because water is the least understood and most complex substance on earth, if not in the universe.
The effect has been documented in peer-reviewed journal articles, such as D. Auerbach, Supercooling and the Mpemba effect; when hot water freezes quicker than cold, Am. J. Phys. 63 (1995) 882-885 and J. D. Brownridge, A search for the Mpemba effect: When hot water freezes faster then cold water, arXiv:1003.3185v1 [physics.pop-ph] (2010).
Your explanation of the bursting of hot water pipes before cold pipes is self-contradictory and incorrect. You claim that cold water pipes can relieve pressure at one end through toilet fill valves while hot water pipes can relieve pressure also at one end through temperature/pressure relief valves, but somehow which end makes a difference.
Water freezing in a pipe is a local phenomenom - it begins at a precise location and the ice plug expands as it freezes isotropically - in all directions. The slight expansion (10% max) has almost no effect on the water pressure on either side of the plug, but is more than sufficient to stretch a copper water pipe to failure as it expands perpendicular to the pipe walls.
Here's why it matters which end the pressure relief is located: If there is no pressure relief between the ice plug and the last fixture on the plumbing run, the pipe will burst. The PT relief valve at the hot water tank can't relieve the pressure buildup between the ice plug and the last fixture on the run.
Bill Rose measured elevated water pressures between the freeze and the end of the pipe due to lengthwise expansion of the water in the pipe. the ice crystals froze from the perimeter of the pipe and grew in towards the center (like those donut shaped ice cubes at hotel ice makers.) There was very little expansion due to ice on the walls of the pipe, the failure was due to water pressure between the ice plug and the fixture. Pipes with poorly installed insulation with intermittent gaps at the connections and elbows saw more damage that pipes with continuous insulation or none since pipes with no insulation tended to fail at a single point and intermittent insulation had the potential to create multiple failures necessitating multiple repairs.
A simple solution would be to add a Tee in the 3/8" pex line under the furthest sink and allow a small amount of cross connection between hot and cold at that point (between the shut off and the vanity faucet hot and cold. You could limit this with a valve or a piece of rubber in the tee fitting so you would still be able to draw cold water at the sink but, if the hot pipe had a pressure build up due to ice, the pressure could push water through the tee into the relief at the toilet fill valve.
Martin,
According to your theory, cold water pipes would burst upstream of the ice plug and hot water pipes would burst downstream of the plug. But that's not where they burst and certainly doesn't explain the difference in rate of bursting.
It's not the widely-disbursed slight increase in water pressure than makes any difference, and the very slight expansion of ice will have almost zero effect on the static water pressure in a pipe.
1/2" copper M pipe is rated for a working pressure of 500 psi, but expanding ice can exert a local pressure of 40,000 psi (enough to crack rock and lift buildings).
Pipes burst at the ice plug, not where there is still liquid water to absorb the very slight decrease in volume.
Hot water pipes burst before cold water pipes because they freeze sooner. Just ask Mpemba.
Cold water pipes don't burst upstream from the ice plug because pressure relief upstream of the ice plug is provided by either (1) the plumbing system's pressure tank (in the case of a rural home) or (2) the nearly infinite size of the municipal water system (in the case of an urban home).
I regularly freeze liquid foodstuffs in glass jars in my freezer, and seldom have had one break. But it is absolutely necessary to leave plenty of headspace between the liquid/ice and the jar cap. Usually a "volcano" forms on top of the ice as the last little bit of liquid freezes in the center of the jar, which has no place to expand to except up towards the lid. If there is not sufficient headspace this "volcano" will press against the lid of the jar and break out the bottom of the jar. While the liquid is freezing there appears to be minimal radial pressure of the ice against the jar as long as there also is liquid that the ice can expand into. But there must be some radial pressure, for when I freeze liquids in squareish plastic containers they do bulge out.
Pipes have burst everywhere in Adirondacks camps. We install compression fittings at the new locales and undue them next Fall closing.
As to hot water... heating water changes the dissolved gas quantities yes? What effect does that have on all these prior discussions?
If someone can link me to a study (Rose's?) that demonstrates conclusively that water pipes burst at places other than the ice plug, I'd like to see it.
The multiple web references to this alleged "fact" all seem to come from the same source, a publication of the Institute for Business and Home Safety titled Freezing and Bursting Pipes (http://www.ibhs.org/natural_disasters/downloads/freezing.pdf). This publication refers to research conducted by the Building Research Council at the University of Illinois (Bill Rose's hangout), which also claims that wind chill can play a major role in accelerating ice blockage in pipes. But wind chill is the subjective experience of cold on a human body in a windy environment (making it feel colder than the air temperature), while wind will accelerate cooling but cannot lower the temperature of an inanimate object below the air temperature.
Since the actual burst strength (as differentiated from working pressure) of a ½" copper water pipe is 6,000-8,000 psi (2 to 3 times the compressive strength of concrete), one would expect to see compression fittings on supply risers and washerless faucets rupturing before a pipe would burst if downstream water pressure were the culprit.
"But wind chill is the subjective experience of cold on a human body in a windy environment (making it feel colder than the air temperature), while wind will accelerate cooling but cannot lower the temperature of an inanimate object below the air temperature."
Not germane to the discussion of freezing pipes, but wind can chill water below the ambient temperature via evaporative cooling. Another magical quality of water... :)
Hello all,
The pipe bursting report has recently been committed to pdf. It is in 3 parts, approx 30MB. If GBA could provide an upload ftp site, I'd be glad to let you have it. It should go a long way toward answering many of the questions posed here.
One unfortunate part of the report is our use of the term "wind chill". It was used in the report to show that, under our field conditions, we were only able to create ice blockage with cold temperatures plus a fan to lower the film resistance. The meterology use of the term is more important, and includes evaporation, not just film resistance reduction. Evaporation was not occuring in our studies, of course. Poor choice of term. Can't go lower than ambient.
The report should be convincing that the burst occurs due to elevated fluid pressure due to the piston-type action of a growing ice block against a confined water quantity. The burst will occur at the weakest point downstream from the blockage, which is usually the pipe. And if it's copper pipe, it's likely to occur where pipe is least ductile, and that is where it is coldest--at the margin between the ice and the water. Toilets can act as pressure relief valves. We're pretty sure, but not positive, that that's why repairs are more common in hot water lines than in cold.
"Toilets can act as pressure relief valves. We're pretty sure, but not positive, that that's why repairs are more common in hot water lines than in cold."
Thanks, Bill. If you can e-mail me the pdf, I'll determine whether GBA can somehow put it online (with your permission, of course.)
"Toilets can act as pressure relief valves. We're pretty sure, but not positive, that that's why repairs are more common in hot water lines than in cold."
Too bad you didn't shut off the toilet supply valve. Then you would know. Or maybe the test wasn't done on an actual plumbing system?
Perhaps even more fascinating than the study on bursting pipes is the 2008 Documentary, Water: The Great Mystery. I just ordered a DVD and I'll offer a review once I see it.
http://www.voiceentertainment.net/movies/watermovie.html
"Water is the driving force of all nature."
- Leonardo da Vinci
"We live by the grace of water."
- National Geographic Special Edition, Nov. 1993
"Water is H2O, hydrogen two parts, oxygen one, but there is also a third thing, that makes it water and nobody knows what it is."
- D H Lawrence
"This film is about water, the most amazing yet least studied substance. From times immemorial, scientists, philosophers and theologians tried to understand its explicit and implicit properties, which are phenomenal, beyond the common physical laws of nature."
"Witness recent, breathtaking discoveries by researchers worldwide from Russia, Kazakhstan, Switzerland, Israel, the USA, Britain, Austria, Japan, Argentina, China and Tibet."
"The arguments expound upon unexpected and challenging assumptions enlightening many years of research to open humankind to new horizons, such as the applications of structured water in agriculture, or the use of water in treatment for the most serious diseases and more."
"The Geography of the film spans the globe. The implications go beyond the solar system, suggesting that water has the ability to convey messages faster than light, perhaps linking water with the absolute. Water is so unique, and so profound, its miraculous properties are still awaiting to be discovered."
Great thread!
Whenever I hear or read a report on new developments in a particular field I am often surprised to find a line like "...it turns out that the processes are far more dynamic than previously thought".
Who would have guessed that life just isn't that simple ;-)
I will Second that !!
In a similar vein...my late uncle Murray, MD & PhD from Harvard, life-long practicing physician, life-long professor of Medicine, on President Kennedy's medical advisory commission, traveled the world to almost every international medical conference (including behind the Iron Curtain during the Kruschhev years), married to a physician, three children who each went into medicine...
When he was dying of cancer and realized that it was incurable by the best that modern medicine could offer (he even tried experimental neutron radiation therapy), went home to learn macrobiotic diet and transcendental meditation so that - if he could not prevent his dying - he could at least learn to die well. And the last thing he said to me, after I read him The Physician, a novel by Noah Gordon, was "We just don't have any idea what we're doing".
It took him a lifetime of dedication to science and medicine to realize the depth of his ignorance. Science and rational thought, as I have hitherto suggested, are not The Way, but merely useful tools to assist us on the Journey.
From that wise guy, Albert Einstein:
"We should take care not to make the intellect our god; it has, of course, powerful muscles, but no personality."
"The intuitive mind is a sacred gift and the rational mind is a faithful servant. We have created a society that honors the servant and has forgotten the gift."
"My religion consists of a humble admiration of the illimitable superior spirit who reveals himself in the slight details we are able to perceive with our frail and feeble mind."
"Every one who is seriously involved in the pursuit of science becomes convinced that a spirit is manifest in the laws of the Universe – a spirit vastly superior to that of man, and one in the face of which we with our modest powers must feel humble."
"Every serious scientific worker is painfully conscious of this involuntary relegation to an ever-narrowing sphere of knowledge, which threatens to deprive the investigator of his broad horizon and degrades him to the level of a mechanic ...It is just as important to make knowledge live and to keep it alive as to solve specific problems."
I tend to find the reflective qualities of water most interesting. Hot or cold there is a distinctive difference. Maybe memory plays a role here? Cold still water reflects perfectly, while distortions seem to occur in still warm water.
We restored a vintage 1910 reflecting pool for a client that had lived on the property for 60+ years. The pool was originally designed as a part of the landscape design to reflect elements of the gardens to a dining area veranda.
The reflecting pool was shallow, (3') and the owner wanted to use it as walk therapy for himself and his wife. They were in their eighties and had us add a propane heat system to the pool as well.
The point of the original design of this pool was to provide a beautiful reflection of the garden's seasonal changes. It was not to be used for swimming etc. The inside was a dark green ceramic tile blend and this thing was hugh, 80'X 28'. I'm pretty sure that the original designers of this type of reflecting pool had to use some sort of science to achieve what appeared to be perfect reflection.
The owners and I both noticed a substantial difference when the pool was heated. ( no chemicals allowed).
Anyhow ~ maybe something else to reflect on.
Thanks for all the great info!
Roy Harmon
When a ripple turns back to stillness~ memory?
My apologies.
Timmy O'Daniels,
With such a wonderful name, I don't know why you wouldn't share it. Apologies as well, but there's been a problem on this site with people flaming under the guise of anonymity or pseudonymity. I didn't suspect you were of that type but - just as I've made it a point of principle not to habituate any real-world venue that isn't smoke-free - I'm similarly trying to ignore cyber-world "conversations" with nameless souls. The Virtual World is a surreal enough place as it is.
I only briefly passed through the Pacific Northwest, and that was 36 years ago, so I have only a "warm water" memory of the climate. But I'll share what I offered on another thread, which I believe is a good rule of thumb:
"To Build a Better Home,” published by the APA-Engineered Wood Association, 2002:
"In areas like the Southwest that receive low rainfall (less than 20 inches annually), a housewrap or building paper should offer sufficient water resistance protection, according to most building experts. In areas that experience moderate amounts of rainfall (20 to 40 inches annually), protection against rain penetration should include an enhanced housewrap. And for wet and/or humid climates, coastal areas and hilltop exposures receiving high (40 to 60 inches annually) or extreme (60 inches or more annually) rainfall, a ventilated rainscreen assembly is recommended; a rainscreen system is also advised for areas that receive high winds in addition to rain. Rainscreen systems are recognized by leading building trade associations for their effectiveness in controlling rain water intrusion into wall assemblies in areas of high and extreme rainfall."
This advice is similar to what is proposed in the HUD PATH manual "Moisture-Resistant Homes: Best Practices Guide for Builders and Designers", which also adds overhang ratio and site-specific exposure into the formula - more roof overhang per storey, the less protection is needed for the walls beneath (A Canadian study also concluded that the overhang ratio was a primary determinant of longevity of structure).
Of course, still water was the original mirror to humanity (and, no doubt, other sentient beings). How many of the myths of our culture involve a protagonist seeing their reflection in a quiescent pool?
Interestingly, Native Americans understand that the Stone People (the bones of the earth) are the keepers of the memory of all that has been. They are, after all, the ones who persist almost unchanging for the longest time. It's not coincidental that, for modern culture - including we who spend far too much time in cyberspace - all our digital memory is contained within silicon chips: rocks, the Stone People.
The Old Man Speaks
Do not cry tears of sadness for me, only tears of joy.
For I am not gone from this hallowed place;
my spirit remains.
I walk with you now.
The torch has now been passed
You must be the guardians of each other now.
The rocks, the trees, the water, the wind, rain and snow
All carry my life force through these mountains.
No longer do I simply sit here looking down upon you
But now I soar and sing and embrace each one of you.
Feel my spirit and care for this earth as you cared for me.
Protect it, nurture it, and feel restored as you walk among this beauty.
Gaze up on a clear and sunny day and you will see me.
Look into each other's eyes; you will find me there.
I have not died
I am here still
Only now I live
Free.
Excerpted from: "The Old Man Speaks", an elegy to the Old Man in the Mountain, Franconia NH (discovered 1805 - collapsed May 3, 2003) by Edwina Landry, Concord, NH, May 6, 2003
Watts makes a toilet valve designed to be an 80psi pressure relief valve:
http://media.wattswater.com/PF-Gov80M2.pdf
Since we're on the subject, I admire the Fluidmaster Leak-Sentry for the way it stubbornly refuses to waste water: http://www.fluidmaster.com/pdfs/400ls_instructions.pdf
Pretty much every toilet valve is a pressure relief valve. can't honestly think of one that isn't though I suppose its possible.
It's especially an issue in urban areas where street pressure is over 80 psi if your pressure reduction valve goes bad the toilets won't shut off correctly and you'll run up a big water bill.
From the sublime to the mundane (and almost ridiculous). Toilet valves? Can we not think outside the tank?
By the way, a quick reading of the 52-page report on the pipe freezing study that Rose referenced, indicates that this "definitive" study (perhaps the only one of its kind) on which everyone seems to be basing their conclusions, was a very limited combination of laboratory and "field" study (the field was the Building Research Council mockup buildings, not actual houses).
The phase 1 lab sessions used a freezer with vertical 3/4" iron pipes, while the mock-up phase 2 sessions used horizontal pipes in a simulated unconditioned attic dropping down into conditioned space.
In my somewhat limited field experience, I've yet to see an iron pipe burst (though I know they can) and all the freeze-burst copper pipes I've repaired were horizontal runs.
While most of the limited number of pipe bursts in the study occurred distant from the ice plug, even in the phase 1 sessions they had a pipe burst at the plug and one of the three bursts in phase 2 also occurred at the plug. They acknowledge, because of this mixed result, that they cannot determine which type of burst is more common.
If the freeze bursting of copper pipes in residential settings were primarily caused by high pressure in the unfrozen section between the ice plug and the fixture, one would expect two outcomes:
1) the supply risers (those flexible 3/8" chromed copper pipes with compression nuts) would separate or rupture or the faucets would be damaged
2) the copper pipes would expand to the point that a repair fitting would no longer fit over the outer diameter
I have never seen a fixture supply riser or a faucet damaged by pipe freeze-up, and I have always been able to repair frozen and burst copper piping by cutting out the swollen and ruptured section and sweating new fittings within a few inches of the damage.
Thus, my own actual field studies suggests that copper pipes more likely (or almost always) burst at the expanding ice plug rather than downstream.
(And their refutation of the different probability of hot vs cold pipe freeze-up was entirely speculative and had no resemblance to Mpemba effect studies or actual field experience.)
Does anyone else have field experience that would suggest otherwise on either of these "findings"?
Robert Riversong said: “I have never seen a fixture supply riser or a faucet damaged by pipe freeze-up, and I have always been able to repair frozen and burst copper piping by cutting out the swollen and ruptured section and sweating new fittings within a few inches of the damage.
Thus, my own actual field studies suggests that copper pipes more likely (or almost always) burst at the expanding ice plug rather than downstream.”
Bill Rose said: “The report should be convincing that the burst occurs due to elevated fluid pressure due to the piston-type action of a growing ice block against a confined water quantity. The burst will occur at the weakest point downstream from the blockage, which is usually the pipe. And if it's copper pipe, it's likely to occur where pipe is least ductile, and that is where it is coldest--at the margin between the ice and the water.”
…….
Does anyone who has repaired frozen pipes have any idea whether the rupture was upstream or downstream from where the pipe originally froze?
.........
Robert Riversong said: “1) the supply risers (those flexible 3/8" chromed copper pipes with compression nuts) would separate or rupture or the faucets would be damaged”
For pipe of the same material and wall thickness (and the supply risers and the feed line aren’t the same, but may be similar), a smaller pipe (3/8”) would have a higher burst strength than a large pipe (1/2”).
But different pipe sizes and types do NOT have the same wall thickness. ½" copper L has slightly thicker walls than 3/4" copper L. The chrome supply tubes are 3/8" OD and have thinner walls than type L pipe. They also rely on compression fittings to resist high pressure.
The Bill Rose quote is contradicted by the findings in the study.
As both his study and all research about the freezing of water indicates, water supercools before freezing and then warms to 32° as it turns into ice, so the coldest part of the pipe would not be at or near the ice plug.
According to the "Copper Tube Handbook --
http://www.copper.org/publications/pub_list/pdf/copper_tube_handbook.pdf
-- the burst strength of 1/2" L (.040 wall) at room temperature is 7765, while the burst strength of 3/4" L (.045 wall) at room temperature is 5900. It doesn't list the burst strength of 3/8" flexible copper tubing. This demonstrates that smaller pipes can be stronger then larger pipes even if the smaller pipe has a thinner wall. So we cannot yet rule out the possibility that the supply risers can withstand more pressure than the supply tubes even if the supply riser has a thinner wall.
Robert Riversong said: "so the coldest part of the pipe would not be at or near the ice plug."
Surely if not AT the ice plug then certainly EXTREMELY close to the ice plug. It is at the ice plug where the source of the cold is, is it not?
"Surely if not AT the ice plug then certainly EXTREMELY close to the ice plug. It is at the ice plug where the source of the cold is, is it not?"
Besides, it's not the water/ice chilling the tubing, it's the tubing chilling the water/ice.
http://en.wikipedia.org/wiki/Mpemba_effect
http://www.scientificamerican.com/article.cfm?id=is-it-true-that-hot-water
John,
Water, particularly cold water, has to supercool - or drop below 32° in order to initiate freezing, then as it turns to ice the temperature jumps back up to 32° until it's completely frozen (and while it's expanding 10% in volume) because freezing is an exothermic reaction - the water gives up the heat of fusion to its environment, in this case the pipe. So the point at which the ice plug begins to form is, for a while at least, warmer than the still-liquid-filled pipe close by.
Robert,
It seems we are discussing two (or more) questions: :)
1) Does expanding ice exert sufficient pressure to burst copper pipe if the system is not capable of being pressurized by expanding ice. My experience with freezing water in a glass jar suggests to me that the system must be capable of becoming pressurized, at least locally, for the expanding ice to exert significant pressure on the walls of the tubing.
2) If pipes burst due to hydraulic pressurization by the expanding ice acting like a piston, where is the weakest portion of the tubing? You suggested that the 3/8" supply riser tubing would be weaker than the 1/2" or 3/4" supply lines. My incomplete data suggests that you may be wrong - that 3/8" tubing could have a wall thickness of half that of 3/4" tubing (of the same material) and be just as strong. Bill Rose suggested that the weakest part of the system might be where the tubing was coldest and least ductile, which would probably be at the ice plug. Since copper is highly conductive thermally, I might guess that the copper is coldest in the immediate vicinity of the ice plug since that is where the cold source is -- else the ice wouldn't be forming there in the first place.
I've never had to repair a frozen and burst copper pipe. But I might guess that if it was the expanding ice, solely, that was causing the burst, the break might just as often happen on the upstream as the downstream side of the plug. If that were the case half of the breaks would be on the upstream side, and would leak profusely, while the other half would be on the downstream side, and leak very little. If the hydraulic pressure theory is correct then most/all of the breaks would be on the downstream side and would leak very little water -- until the ice plug thawed of course.
Robert Riversong said: "Water freezing in a pipe is a local phenomenom - it begins at a precise location and the ice plug expands as it freezes isotropically - in all directions. The slight expansion (10% max) has almost no effect on the water pressure on either side of the plug, but is more than sufficient to stretch a copper water pipe to failure as it expands perpendicular to the pipe walls."
Actually, ice is plastic under pressure. It will flow from from an area of high pressure to an area of low pressure. That characteristic is what allows glaciers to flow. Another magical quality of water :)
Robert,
I've repaired a burst pipe in a fixture riser. It happened at my mother's house, three years ago. It was 1/2-in. copper tubing -- a horizontal riser under the kitchen sink. She forgot to drain it properly before she went to Florida.
John,
If copper pipe ruptures where it's weakest, that's more likely to be near a fitting where it was overheated during assembly, or where acidic water or high-velocity has been scouring the pipe at changes in direction, or at a manufacturing defect (I doubt that the thickness is uniform), or where galvanic action has been eating the metal, or where soft ("hungry") water has been dissolving the copper, or wherever the water decides is the most convenient place to exit...
The simple truth is: we don't know where the burst might occur in relation to the ice plug and it really doesn't matter. What matters is to keep pipes from freezing and rupturing.
Ice may be plastic over a glaciatic time scale, but it also has enormous adhesive strength and almost unimaginable expansive power, such that it can grab and lift a smooth concrete or wooden pier by the sides and raise a house.
I routinely bring in 5-gallon plastic buckets of ice which are open to the air on the top yet the ice bulges out the bottom enough to break it. The inside surface of the drywall buckets is at least as smooth as a copper pipe and yet the ice adheres to it sufficiently to break the bottom of the bucket even though it can move plastically toward the open top.
Water already is, except in the specific cases of flood, drowning and hyponatremia, an ally of the living human being and always will be. It is, however, a relentless enemy of the inanimate materials from which we (try to) make our shelters. It is the chief agent by which time frustrates our efforts in this regard. It destroys the inorganic by erosion and corrosion and turns the organic into food for a myriad of lifeforms. Its physical properties give it an insidiousness which taxes our ingenuity and punishes errors of thought and execution. Of all the enemies of our physical structures it is therefore accorded the greatest respect and receives our greatest attention. If (and I know there is considerable debate on this) we want to make our dwelling durable it is only prudent that much of our attention should be spent on how we deal with both the water we need and the water we don’t.
Of course we should not forget the warning given by Danish mathematician and inventor Piet Hein
GBA has posted the research report on frozen pipes mentioned by Bill Rose.
If anyone is interested, the report is posted here.
Perhaps more correct to say that water is the primary ally of Life and an enemy only to humanity in so far as we ignore the laws of Life (natural law). It is not an enemy of materials but rather an enemy of humanity's hubristic attempts defy Nature in our clever designs and our foolish use of materials.
From Nature's perspective, water alchemically transforms rock into soil, waste into food, and death into new life. It is only when we modern, advanced (sic) humans believe we can defy and outsmart Mother Nature that we turn a holy ally into a demonic force. [The real message of the Garden of Eden story is that evil and suffering did not exist in the world until we turned our backs on Nature and chose to leave the Garden of Earthly Delight – this was the "original sin".]
The reason that "problems hit back" is that we approach them with arrogance and ignorance rather than a humble and intuitive (common sense) appreciation of our place in the scheme of things.
Why Things Bite Back: Technology and the Revenge of Unintended Consequences by Edward Tenner, 1997 (historian of science and visiting researcher at Princeton) gives us myriad case studies of how technological fixes often create bigger problems than the ones they were meant to solve in the first place.
Even when used to better the world, technology fosters unforeseen, often unpleasant consequences that Tenner calls "revenge effects." For example, air-conditioned subways raise platform temperatures by as much as 10 degrees F; flood control systems encourage settlement of flood-prone areas, inviting disaster; 6% of all hospital patients become infected with microbes they encounter during their stay (and modern medicine is the leading cause of death in the US).
Oil spills, erosion of beaches, back injuries, athletes' illegal use of steroids and mass extermination of bird species on the world's islands by ship-hopping rats mark this saga of bewildering, often frustrating change. Tenner's cautionary conclusion: revenge effects demand ingenuity and brainpower as technology continues to replace immediate life-threatening problems with slower-acting, more persistent ones.
But if the attention leads only to more strident methods for defying Nature's Laws, leading to an increasing potential for unintended consequences, revenge effects or "blowback" - in other words, "solving" the problems with the same technologies and mindset which created them - then we have not demonstrated a capacity for respect, but merely the audacity of fools.
When we talk about water freezing to burst pipes, what role would metals, salts, minerals or other water treatment type contaminants play regarding A. pipe strength B. temperature change? Is this even a factor to consider?
A more relevant factor is the pipe material. Copper pipe, which has long been the standard for quality water supply systems, is becoming problematic because of the increased acidity of our water supply, the increasing prevalence of water softeners (low dissolved solids), and high dissolved oxygen - all of which conspire to dissolve copper and raise the concentration beyond the EPA threshold of 1.3 ppm. Copper ingestion is now associated with flu-like symptoms, kidney damage, Wilson's disease, learning deficiencies in adolescents, and Alzheimer's disease. It is not known whether it is carcinogenic.
In addition to the scouring effect of piping with lots of angular changes of direction, there is evidence that such "mechanized" water loses its energetic qualities. In nature, water flows in smooth curving channels.
While there are also problems with domestic water supplied through plastic piping (such as MBTE and other contaminants, that might be triggered by the interaction of chlorine with the plastic), PEX water supply piping doesn't scale or pit or react galvanically, allows the water to flow more naturally thus reducing turbulence and noise and possibly improving its energetic qualities, and is relatively resistant to freezing and rupture. It also has 85% less embodied energy than copper per unit volume and far fewer joints to leak.
Because PEX requires less labor to install, it is more appropriate for "home run" or parallel supply systems, with a small-diameter individual hot and cold line to each fixture, thereby eliminating pressure drop and reducing wait time for hot water.
Research has shown evidence that PEX pipe is susceptible to permeation by outside contaminants such as pesticides, oil, gasoline, benzene and termiticides (if used outdoors) and had the strongest biofilm formation and the strongest initial promotion of the growth of Legionella bacteria.
As with most technical choices, this one may be the lesser of two evils. Toss a coin.
I just received and watched the documentary Water: The Great Mystery.
From the Jacket:
This film is about water, the most amazing yet least studied substance. From times immemorial, scientists, philosophers and theologians tried to understand its explicit and implicit properties, which are phenomenal, beyond the common physical laws of nature.
Witness recent, breathtaking discoveries by researchers worldwide from Russia, Kazakhstan, Switzerland, Israel, the USA, Britain, Austria, Japan, Argentina, China and Tibet.
The arguments expound upon unexpected and challenging assumptions enlightening many years of research to open humankind to new horizons, such as the applications of structured water in agriculture, or the use of water in treatment for the most serious diseases and more.
The Geography of the film spans the globe. The implications go beyond the solar system, suggesting that water has the ability to convey messages faster than light, perhaps linking water with the absolute. Water is so unique, and so profound, its miraculous properties are still awaiting to be discovered.
For those who are open to a scientific/philosophical/spiritual exploration of the magic and mystery of the most common compound in the universe and the most ubiquitous substance on earth, I would highly recommend this film.
"The Universe exists as a single perfect organism. All of its parts, including us and our earth, are inseparably bound together by huge streams of information. On our planet, water plays the key role in how the information is exchanged. It is the medium through which all nature is governed. Each of us is both link in an endless chain of information transmission and a source of information. Every one of our actions – a thought, an emotion, an uttered word – separates from us and becomes part of the informational environment."
From the film:
In 1472, Abbot Karl Gustinzis was imprisoned by the Inquisitor after being accused of causing a prominent woman to fall ill. He was given only a crust of bread and a dipper of stinking water each day, but after forty days was seen to have gained health and strength (encouraging the belief that he was in league with dark forces). The abbot confessed under torture that he had recited a prayer over the water each day, thanking the Lord for bestowing trials upon him, upon which the water turned fresh and clear and tasted bland.
In the winter of 1881, the sailing ship Lara was on a voyage from Liverpool to San Franciso when, on the third day, a fire broke out and the passengers abandoned ship on lifeboats. The captain and others soon ran out of fresh water and began to suffer thirst and dehydration. They explained how they survived three weeks by dreaming about fresh water, imagining that the blue ocean around them was the emerald green of fresh water, and then scooping it up to drink – and it tasted exactly like the fresh water they had imagined.
There is a legend among the Persian Sufis: Once upon a time, a wise man said that all the water in the world would disappear and the water which replaced it would cause anyone who drinks it to lose their mind. Only one man took the prophecy seriously and collected all the old water he could. As it was told, all the water dried up and was then replaced by more. When people thirstily drank this new water, they went mad. But the one who had heeded the warning drank only from his own supply and kept his sanity. He was the only sane one among the madmen and therefore he was called crazy. So he poured his old water onto the ground, drank the new water and lost his mind, and the madmen decided he had regained his sanity.
I have not been able to download the Jeffrey Gordon paper.
(problem may be with my computer)
Concerning Pipe failure....
I don't remember anyone in this thread mentioning "Cold Edge Effect"
There is fascinating illustration in Bill Rose's Water Book.
I have not been able to find a "cold edge efffect" illustration "on-line"
Does anyone have a good link?
I am guessing that One of the reasons that Passivhaus promotes "thermal bridge free" construction is related to "cold edge effect".
"In the winter of 1881, the sailing ship Lara was on a voyage from Liverpool to San Francisco when, on the third day, a fire broke out and the passengers abandoned ship on lifeboats. The captain and others soon ran out of fresh water and began to suffer thirst and dehydration. They explained how they survived three weeks by dreaming about fresh water, imagining that the blue ocean around them was the emerald green of fresh water, and then scooping it up to drink – and it tasted exactly like the fresh water they had imagined. "
...
Apparently humans can live for a time on sea water. In 1952 Dr Alain Bombard spent 65 days crossing the Atlantic from the Canary Islands to Barbados in a raft. He drank only sea water and what rain he could collect. (The Voyage of the Heretique)
Also, William Willis rafted from S America to Samoa (1954) and from S America to Australia (1964). On both trips his fresh water supply was compromised early in the trip, and he also survived on sea water and rain. (The Epic Voyage of the Seven Little Sisters: A 6700 Mile Voyage Alone Across the Pacific)(An Angel on Each Shoulder)
Cold water in a tea pot will always boil much faster than water at warm or room temp.
Actually, the "cold water boils faster" story is false. Cold water will absorb heat at a higher rate than hot water (delta-T phenomenon and internal convection), but once it reaches the same temperature as the hot water they both take the same amount of time - from that point on - to boil.
However, it's been established experimentally - at least at the quantum level - that "a watched pot never boils".
This is due to the quantum Zeno effect, earlier called the Turing Paradox:
"It is easy to show using standard theory that if a system starts in an eigenstate of some observable, and measurements are made of that observable N times a second, then, even if the state is not a stationary one, the probability that the system will be in the same state after, say, one second, tends to one as N tends to infinity; that is, that continual observations will prevent motion …
– Alan Turing as quoted by A. Hodges in Alan Turing: Life and Legacy of a Great Thinker p. 54
"For example, suppose you are watching a quantum system and attempting to determine just when it undergoes a transition from one state to another. To make this concrete, think of a an imaginary "quantum pot of water" being heated on the stove. The transition is for the water to go from the calm state to the boiling state. We all know that pots of water boil, given a few minutes or so. You would certainly think that the watched pot would also boil. It turns out that, because of the vigilance of the observations, the transition never occurs; the watched pot never boils This experiment was reported in the popular science magazine Discover.
See Freedman, David H. "Weird Science." Discover Magazine. Vol. 11, No. 11, pp. 62-68
Without a significant amount of freshwater, a person cannot survive on seawater.
Human blood has 0.9% salinity, while seawater is 3.5% salt. Drinking water that's almost four times saltier than our body can tolerate requires eliminating large quantities of water, through the kidneys, to eliminate the excess. That quickly causes dehydration, electrolyte imbalance and kidney overload and failure. Since human kidneys excrete urine that is slightly less saline than seawater, they have to eliminate more water than is consumed in order to eliminate the salt and maintain the body at "normal saline" conditions.
However, the common knowledge that a human being can survive no more than about 3 days without water is not true, unless one is doing physical work and/or exposed to dehydrating environmental conditions. I know this because I have gone 6 days and later 10 days with no water or food intake and lived to tell the tale (a mentor of mine went 14 days).
I recently received, from John Brooks (thank you), the Luna Leopold book "Water", which is part of the old Life Science Library, published in 1966. Fortunately, water hasn't changed since then and the earth now has the same amount of water it had at its birth.
As I read through it, I'll share some of the fascinating information about water. Here are some:
Water is ubiquitous: the surface of the earth holds 324 million cubic miles of water, while the ground contains another 2 million cubic miles (36 times as much as on the dry land surface) and the atmosphere holds 3100 cubic miles more. One cubic mile is 3.38 million acre-feet of water (which is equivalent to Connecticut under one foot of water). If all the atmospheric water fell at once, it would cover the earth with barely an inch of rain. Once every 12 days all the water in the atmosphere cycles to earth and is replaced. Deep groundwater, however, may not return to the hydrologic cycle for decades, centuries or millennia and there is water in the center of the earth that has been there since the earth was formed.
Of the 95,000 cubic miles of water that cycles through the atmosphere annually, 80,000 cubic miles rises from the oceans. The other 15,000 cubic miles evaporates from the land and from the transpiration of plants. Most of that, some 71,000 cubic miles, falls back into the oceans, leaving 9,000 cubic miles of water to replenish the land and slowly return to the seas.
The greatest evaporation occurs, not at the equator which is often covered in cloud, but between 15° and 30° latitudes where dry winds scour the earth. The Red Sea loses 11½ feet of water per year, which is why it is so saline.
Precipitation can range from an annual average of 460 inches on parts of Hawaii to 1.7 inches in Death Valley. The average annual rainfall in the US is 30 inches, but the variance is dramatic, with the western Cascades getting 150 inches because of orographic and coastal effects.
Water, in both seas and atmosphere, moves great amounts of energy around the earth. The Gulf Stream carries warm tropical waters up to the British Isles and Norway, providing a temperate climate to a geography at the same latitude as frigid Labrador. In a current that is 20°F warmer than the surrounding ocean, each cubic mile of water carries heat equivalent to the efficient burning of seven million tons of high-quality coal.
More than a million years ago (the Pleistocene era or ice age), the earth's temperature fell by three or four degrees and so much water was locked up in ice thousands of feet thick (as far south as St. Louis) that the ocean levels dropped 300-400 feet. The last glaciers retreated only 10,000 years ago.
Lakes are short-lived anomalies in the hydrologic cycle, which begin to die as soon as they are formed, either by sedimentation or evaporation. Death Valley was once under 180 feet of water and the Great Salt Lake was originally almost eight times larger (Lake Bonneville) which had no outlet other than evaporation.
Water maintains itself with such tenacity that, until 225 years ago, it was thought to be an indivisible element rather than a compound.
Hydrogen and oxygen easily bond together, releasing in the process significant energy. When 14 ounces of pure oxygen and 114 ounces of pure hydrogen combine to form 1 gallon of water, it provides enough energy to light a 60 watt bulb for 270 hours (16,200 watt-hours, or 55,274 BTUs). This energy release was the power source for the Gemini V spacecraft.
The tenacity of water is from the covalent bond between the dissimilar atoms. While a hydrogen atom has only one electron, it has room for two in its outer orbit. The oxygen atom has six electrons with room for eight. So two hydrogen atoms and one oxygen atom complete each other's electron "shells", creating an enormously stable compound that resists decomposition.
The configuration of the water molecule is lopsided, with the hydrogen atoms on one side of the oxygen atom and at a 105° angle to each other. This creates a dipole, like a bar magnet, with the hydrogen side having a positive charge and the oxygen side of the molecule being negatively charged. And this explains water's power as a solvent.
Many compounds, such as table salt (sodium chloride) are held together by simple electrical (ionic) attraction. If water inserts itself between the two ionic molecules, its dipole nature will partially cancel the electrical attraction and separate the ions. Water is, in fact, such a near perfect solvent that pure water may not exist in nature. Though water is repulsed by organic compounds, it is attracted to and a solvent for approximately half of all chemical elements. Even rain dissolves atmospheric gasses as it condenses and descends. All water on earth is an aqueous solution. Sea water is a highly concentrated one, with hundreds of dissolved substances.
Because water is the only substance on earth that becomes less dense as a solid, ice floats and forms an insulating layer on the top of large bodies of water. If this were not so, all lakes and oceans would become frozen from the bottom up, with perhaps a thin layer of liquid on the top during the warmer seasons. Not only could life not persist in the water, but the land surface would have daily temperature swings of hundreds of degrees and parched, searing winds would blow over the earth, since it's the ability of liquid water to absorb and slowly release heat that maintains a relatively constant terrestrial temperature.
In fact, the latent heat of ice is so high, that the heat released by a pound of water in the form of ice is enough to melt eight pounds of iron. A bucket of water in a greenhouse on a freezing night will release enough heat as it turns to ice to keep the greenhouse above the outside temperature. Ice cubes chill a drink, not because the ice is cold, but because the melting ice absorbs so much heat from the water. Turning liquid water into steam requires five times as much heat as it does to turn ice into water, and the same amount is given off when water vapor condenses back into liquid water.
Because of this extraordinarily high latent heat, each droplet of water or molecule of water vapor in a cloud is a bundle of energy that creates global climate and local weather. As cumulous clouds grow on a warm summer day, the rising water column releases heat as it condenses into cloud, creating hurricane-force convection within the cloud. The amount of energy released during a run-of-the-mill thunderstorm is equal to that of a 120 kiloton atomic bomb, and there are 10,000 thunderstorms every day. A small hurricane can whirl five billion tons of water through the atmosphere.
In most compounds similar to water, the liquid range gets lower and narrower on the temperature scale as the molecular weight decreases. Water, however, the lightest of them all, has the highest and widest range.
The reason that ice is less dense than liquid water is that its dipole nature allows each hydrogen pole to bond to two oxygen poles and form stable tetrahedral crystals at cold temperatures, which seek out other such crystals and form the characteristic octagonal snow crystals. Inside these tetrahedrons is empty space, which makes ice very light and "airy". When ice receives energy and melts, some of the hydrogen bonds are broken and the crystalline structure begins to collapse, allowing a tighter packing of molecules. This increase in density continues until 39°, at which point the molecules become energetic enough to move apart so the water becomes less dense with increasing temperature, as do most substances.
These hydrogen bonds are the most tenacious of all molecular bonds, which accounts for the great amount of energy required to break water molecules apart. This tenacity also accounts for the strange ability of water to climb upwards against gravity, called capillarity. Oxygen and nitrogen atoms in a container bond to the hydrogen atoms in water, allowing the water to climb up the edge, while the strong internal hydrogen bonds maintain a high surface tension, drawing the surface of the water column toward the edge (creating the meniscus, or curvature of the surface). As molecules continue to be drawn up the container, more surface molecules are drawn toward the edge, creating a sort of hand-over-hand climb up the wall until the strength of the hydrogen bonds are balanced by the force of gravity. Because capillarity is a gravity-limited phenomenon, water in very narrow tubes can rise to incredible heights (as much as 450' inside the lumens of a tree).
Robert,
You seem to be extremely Verbal and Visual.
I am more on the Visual side.
The Leopold Book is not-so-hard to read AND it has lots of good illustrations.
Nice for people like me.
I am still trying to make myself "really small" so that I can visualize how water vapor moves thru building materials.
You have to be really small AND bi-polar ;-)
Dan,
We weren't talking about rainscreens, which I happen to believe are becoming faddish and are overused and often unnecessary. So I have to assume that your sudden interest in this dormant thread has to do with a financial relationship to Stuc-O-Flex or WaterWay rainscreen drainage mats.
Sorry, but no covert advertising allowed here.
[edit: by the time I posted this response, after checking out the YouTube and corporate links, the offending post disappeared. Must be more of that Water Magic]
"Because water is the only substance on earth that becomes less dense as a solid, ice floats and forms an insulating layer on the top of large bodies of water. If this were not so, all lakes and oceans would become frozen from the bottom up, with perhaps a thin layer of liquid on the top during the warmer seasons. Not only could life not persist in the water, but the land surface would have daily temperature swings of hundreds of degrees and parched, searing winds would blow over the earth, since it's the ability of liquid water to absorb and slowly release heat that maintains a relatively constant terrestrial temperature."
So I guess frost heave is a small price to pay for, umm, enabling life on earth?
I guess that depends on whether you think life on earth has been a good thing or not.
If humanity as we know it represents the pinnacle of the evolution of life on earth, what does that say about the value of the whole evolutionary enterprise? Could it have been a mistake?
I've been studying the phenomenon of thermosiphoning. In this case, water acts as though it is intelligent.
We've learned that if you pipe two tanks together at the top and bottom, they will both stay pretty close in temperature even while you add and subtract heat from just one of them. This is handy if you have a customer who needs more storage for their solar system. You can add storage without a pump or controls. The heat naturally flows in the direction you want it to.
So I'm taking this intelligence and trying to prevent some pipes from freezing using a thermosiphon. I'm a little worried that the liquid water will start to get a bit confused when it reaches the mid 30's, where it's density starts increasing. That problem, combined with increasing viscosity, just might cause the water in the pipes to sit there and get colder instead of flowing down and back into a storage tank.
Has anyone seen any studies on this type of cold water thermosiphon?
Speaking of intelligent water for heat transfer, check out Dan Holohan's books on steam heating and how 19th century man had unlocked most of steam's darkest secrets, only to have 20th century man forget them all.
"Progress" is mostly an act of forgetting what we once knew. On that continuum, we will have achieved the greatest "progress" when we know nothing at all.
Thermosyphoning, of course, requires a significant delta-T, or difference in density and buoyancy. So the only potential problem with cold water thermosyhponing would be an insufficient density difference, and since water below 39.2° is less dense, thermosyphoning for temperature equilibrium is not possible below about 40°.
"If humanity as we know it represents the pinnacle of the evolution of life on earth, what does that say about the value of the whole evolutionary enterprise? Could it have been a mistake?"
A mistake? From whose perspective? Not a mistake for the cockroaches. They don't give a damn one way or another.
And how, exactly, do you know that?
I can easily imagine that cockroaches are not particularly pleased with the evolution of humanity, their primary adversary and the creature that won't even allow them the crumbs of civilization.
Just Trying to imagine how incredibly small a water molecule is.
A single molecule is about 0.15 Nanometers wide
What's perhaps even more astounding is that today's dime (or dollar) has shrunk to only 4% of its 1774 value, and almost all that shrinkage occurred since the Federal Reserve Banksters were put in charge in 1913, making every dollar issued into a debt obligation to the bankers.
In the natural world, water is the universal solvent. In the artificial world, greed is the universal solvent, continuously eating away at the foundations of civilized life.
Great Question.
I've been wondering this myself for decades. Water should be an allie to builders and developers, not a hassle. It needs to be dealt with in the details of construction and collected and kept clean so it's not running down, from house to house and eventually to where it's no longer fresh water.
This is why I'm a big fan of metal roofs that don't pollute the water from the get go, rain screens that allow a wall to shed the water that will undoubtedly gain entrance to any wall system at some point, and water catchment systems. Collector ponds that manage the water can aid in retention, alleviate flooding and run off, restore displaced habitat and actually add value to the finished product. And water is a resource we need to conserve. It is beyond me how any responsible builder/architect could not incorporate clean water retention and rain screen details. Especially in the northwest (WA) where I live.
Conserving water in and around our homes is helpful, but most of the fresh water that we consume is for the agricultural and industrial sectors that support our highly extravagant and wasteful lifestyles.
Eating meat, for instance, requires enormous amounts of water. It takes 1800 gallons of water to produce a pound of beef, and a typical hamburger takes 630 gallons to make. A cup of coffee requires 37 gallons of water, a glass of wine requires 1,000 glasses of water and a gallon of milk takes 880 gallons of water.
On top of that almost unimaginable wastefulness, we throw away 30% of all food produced in the US, which also wastes 11 trillion gallons of irrigation water, which is enough for the total household use of 300 million Americans.
In fact, the single lifestyle change that would do more to save the earth than any other is to reduce or eliminate meat consumption, which would also make us much healthier and dramatically reduce health care costs.
The water cycle of nature has allowed life to flourish, but the water cycles that flow through our culture are destroying the capacity for life on earth.
Since this discussion has organically flowed, like a living stream, from the universal solvent to the dissolution of our economy because of the control of the money system by the Banksters, I wanted to share this astonishing and little known secret:
While every other state in the US is facing high unemployment, drastic loss of tax revenues, excruciating cuts to public programs, and the threat of default, North Dakota alone has 4% unemployment and a budget surplus. How can that be possible? Because they've been Communist for 90 years:
Unemployment is frozen at 9.6 %, with almost 15 million out of work and homes still being lost. Meanwhile, banks sit on unprecedented amounts of cash while their CEOs continue to receive those fat bonuses that incurred the wrath of the nation. And the loans they were supposed to be giving to pump up the economy? Missing in action. The Banksters continue to rule, hoarding our money and refusing to adjust mortgages to keep folks in their homes. Too Big To Fail prevails, as those banks, which are little more than fronts for multinational corporations, anyway, send our money abroad instead of reinvesting in the people and our country’s economy.
Yet while the Fed continues playing parlor tricks to try and stimulate the economy, a much simpler method of igniting long-term economic growth and stability exists in North Dakota. Yes, North Dakota, a state operating with a surplus of cash and unemployment at 4%. In the early 1900′s the economy of North Dakota was agriculture-based, and the farmers there were experiencing serious financial problems that prevented them from buying and selling crops and financing farm operations. Grain dealers from out-of-state controlled prices and kept them artificially low, while farm suppliers continually increased their prices. To no one’s surprise, interest rates on loans climbed.
By 1919 the people of North Dakota had had enough and wanted state ownership and control of marketing and credit agencies, and so the legislature established the Bank of North Dakota. Its mission: to promote the development of agriculture, commerce and industry in ND. The Bank of North Dakota is a public bank that is robustly solvent, with a strong record of financing loans for agriculture, housing and higher education, as well as funding municipal bonds. All tax revenues and fees in the state go into the State Bank, allowing North Dakota to finance construction of roads, bridges and other infrastructure, maintain schools and libraries, and assist local businesses.
The Bank of North Dakota is truly a peoples’ bank that exists for the benefit of the state and its residents, only, with loans made at low interest rates and no bloated, outrageous CEO salaries and benefits that squander funds. And no shady derivatives allowed, either; a novel concept indeed. For 91 years the bank has flourished and North Dakota today is a rare example of economic strength in a sea of debt-ridden states that must slash services and raise taxes to stay afloat, giving a whole new meaning to the term “red states.”
The Bank of North Dakota has now received inquiries from 25 states and 3 foreign governments as a result of its continued profitability. The momentum is building for this sane kind of banking system that works for the people and state instead of the bottom line of banks and shareholders. In the spirit of free markets, a state bank creates competition with private banks, creating lower interest rates on loans for borrowers across the board. One would think the Republicans and conservatives would favor this since, after all, they are the champions of “states rights,” and what is more fundamental than a state’s right to manage its own money?
If governments have the power to create and control the amount of money in circulation, then they do not need to pay banks for their own money creation. The stranglehold of borrowing money creates endless debt that enriches no one but the private banks and gives them incredible power over the nation and its economy.
Hence we see cycles of inflation as debt rises and the need for more money is artificially created. Eventually, the cost of this ever-growing debt becomes too much for the government to pay and the economy goes into a freefall. It is time for the States to show the Federal government how to truly revive our economy.
D
Robert, (and gentlemen?) I'm with you when it comes to eliminating meat consumption. Where would you recommend I get good information not tainted by the food industry about changing to a meatless diet? Does that include no eggs, no dairy products, and no fish? What do you eat, and do you have a formula for a gruel that can take care of nutritional needs through winter until the garden starts producing, for someone that wants to go cold turkey so to speak? My tastes are easy to please, in fact people have told me I have no taste.
Dave,
If you have no taste, it should be a piece of cake;-)
I would suggest not only ignoring the mainstream food industry, but also their hand-maiden FDA (four food groups) and even conventional nutritionists. If you Google "vegetarianism", you'll find an almost limitless source of information. The Vegetarian Times has been around a while. You might explore pure veganism or the ancient macrobiotic principles. But don't take anything as "gospel". Chinese dietary principles are based on the premise of four body types, each needing a different mix of nutrution. And the ground-breaking Diet for a Small Planet shares both the geopolitics and ecology of food and the basics of combining proteins for a complete non-meat diet.
But there are ovo-lacto vegetarians, and fish-eating non-red-meat-etarians, and vegans who don't even eat honey (because it would be exploiting the bees), and localvores, and those who eat only organic happy animals, and healthy conscious opportunivores like myself.
Though I was raised on a conventional American meat and potatoes with vegetables-on-the-side diet, I've gone through stages as a strict vegetarian, a pure vegan, a fish & poultry eater, and a non-dogmatic careful eater of good, local, healthy foods.
Beef uses far more resources than any other common food animal - pork ain't quite as bad and chickens and fish are better yet. But do you eat organic, farm-raised fish that swim in their concentrated excrement or non-organic wild fish – both with doses of mercury? Of course the most honest diet is one that you hunt or forage or grow with your own hands (and probably the most healthy as well).
Some people swear they can't get through the winter without meat, while others have no problem on sprouts. The most dangerous modern food, after red meat, is processed grains and sugars. It's carbs that have been killing us since the start of the age of agriculture and that are responsible for most obesity. Fossil evidence shows dramatic decreases in health wherever grain agriculture started around the world (stunted growth and lots of bone lesions).
Some refuse (or limit) meat to not contribute to environmental destruction, others to minimize world hunger, while some do so for personal health reasons and others for spiritual reasons. My primary spiritual guide said the two best reasons not to eat animals is that we eat their dying emotions (which is literally true in the form of cortisol released during stress) and that we take on their karma - and none of us can afford more karma than we already have.
So there are many paths to eating well, and almost any reduction of red meat will help both your own health and the health of the planet. Organic foods, I believe, are much less expensive than commercial food – because of the savings in health care and the improved quality of life (not to mention the reduced cost to the planet). A good place to start is your local food co-op. They often have educational programs or food tasting events or offer free recipes to help people take the first steps toward a sane diet.
And, as the ancient ones say, a diet of a hundred years starts with the first bite.
[edit: I should add that the only necessary nutrient that's not available in the vegetable kingdom is vitamin B12 - for nerves and brain. But the best source of it is nutritional yeast, which I sprinkle on my buttered toast and use with tamari for the best popcorn in the world.]
Thank you very much Robert. Hope I didn't disturb your dinner. I'd like to add that I am another soul you and Joe Bageant have touched. Keep up the good work. I say this not to glorify, but to let others know that there are people out here paying attention and getting rather fed up with the whole situation. Do you know of any organizations (that are young enough to no need overthrowing yet)where I can meet like minded individuals, who are also trying to escape the matrix?
Robert,
In an act of protest for the unfair, harsh treatment that you and others have been (and are still being) subjected to, I have edited the content from my postings.
What the community rating system has done is most damaging and wrong.
A mere tweek to something this bad will be equally insulting to the injury.
I'm afraid that is what is about to happen tomorrow or Friday.
Dave,
Could I ask where you are and how old you are (Gen Y, Gen X)?
I think anything as defined as an "organization" is likely pretty rooted in the Matrix. But there are some interesting movements, such as Cyber Punk and Steam Punk. And one of my favorite wandering philosophers of that genre is Ran Prieur: His Essays
I published a seminal article of his in 2006 in the Vermont Commons
Ran wrote an essay, in fact, called "How to Eat Better", which begins "What your ancestors ate is good for you." He covers most of the important elements of eating right. He's one of the clearest and most profound thinkers alive today.
Sure. I'm 60 mile southeast of ground zero (D.C.), St. Mary's County, Maryland. I'm fifty years old and wear XX, so I don't really know what Gen I am. Thanks for the links. I see I've got some more good reading to do tonight.
Dave,
I guess that makes you Gen XX ;-)
Any relation to Oskar Schindler (of Schindler's List)?
If you want to get in touch outside this forum, you can reach me at HouseWright (at) Ponds-Edge (dot) net.
We've traced us back to Austria, but no one's gone any further. Thanks for the invite, I shall take you up on that tomorrow. Have a good evening!
I decided to bump this thread because to understand building science you have to understand water. This is a great discussion of water's unique qualities and behaviors.
So this is a good place to ask questions or report some knowledge.
Thanks Kevin Dickson