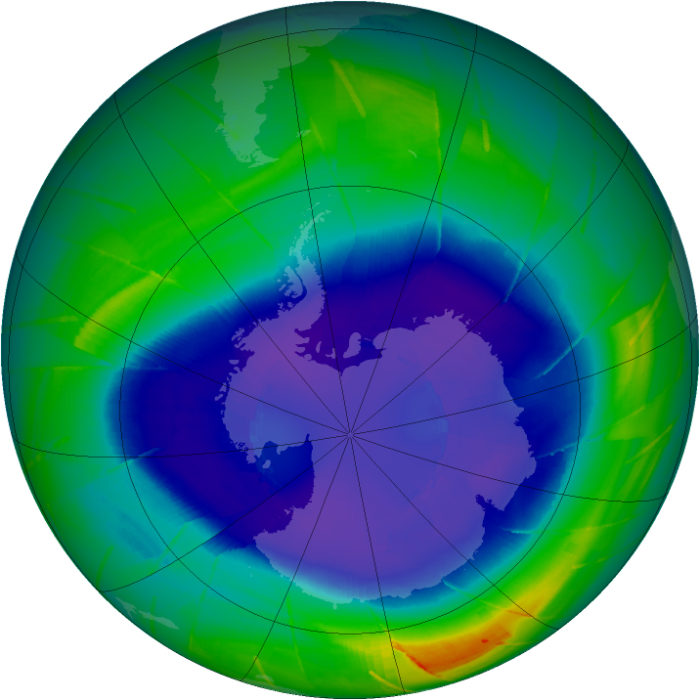
Image Credit: NASA
Did the Montreal Protocol fix the ozone hole? It seemed so. With chlorofluorocarbons (CFCs) and other ozone-eating chemicals banned, many scientists said it was only a matter of time before the ozone layer recharged, and the annual hole over Antarctica healed for good.
But 30 years on, some atmospheric chemists are not so sure. The healing is proving painfully slow. And new discoveries about chemicals not covered by the protocol are raising fears that full recovery could be postponed into the 22nd century — or possibly even prevented altogether.
In mid-September, the United Nations is celebrating the protocol’s 30th anniversary. It will declare that “we are all ozone heroes.” But are we patting ourselves on the back a bit too soon?
The ozone layer is a long-standing natural feature of the stratosphere, the part of the atmosphere that begins about six miles above the earth. The ozone layer filters out dangerous ultraviolet radiation from the sun that can cause skin cancer and damage many life forms. It may have been essential for the development of life on Earth.
So there was alarm in the 1970s when researchers first warned that extremely stable man-made compounds like CFCs, used in refrigerants and aerosols, were floating up into the stratosphere, where they released chlorine and bromine atoms that break down ozone molecules. In the 1980s, Antarctic researchers discovered that these chemical reactions went into overdrive in the super-cold polar stratospheric clouds that formed over the frozen continent. They had begun creating a dramatic “hole” in the ozone layer at the end of each austral winter.
The ensuing panic resulted in the signing of the Montreal Protocol on September 16, 1987. It and its successors have phased out production of a range of man-made chlorine and bromine compounds thought to persist for the several years needed for them to reach the stratosphere. Besides CFCs, they include carbon tetrachloride, hydrochlorofluorocarbons (HCFCs), and methyl bromide, a fumigant once widely used to kill pests.
So far so good. The amount of ozone-depleters in the atmosphere has dropped by more than 10% since peaking in the late 1990s. In response, the total ozone in the atmosphere has been largely unchanged since 2000.
New threats emerge
But in the past five years, evidence has emerged that potential ozone-eating compounds can reach the ozone layer much faster than previously thought. Under some weather conditions, just a few days may be enough. And that means a wide range of much more short-lived compounds threaten the ozone layer — chemicals not covered by the Montreal Protocol.
These compounds are all around us. They are widely used as industrial solvents for tasks like degreasing and dry cleaning. And their releases into the atmosphere are increasing fast.
These new ozone-busters include dichloromethane (DCM), a common and cheap paint stripper, also used in foam-blowing agents and, ironically, in the manufacture of “ozone-friendly” alternatives to CFCs. With emissions now exceeding one million tons a year, the concentration of DCM in the lower atmosphere has more than doubled since 2004. Even so, it has not been regarded as a threat to the ozone layer, because its typical lifetime in the atmosphere before it is broken down in photochemical reactions is only about five months. It should, atmospheric chemists concluded, remain safely in the lower atmosphere.
But that view collapsed in 2015, when Emma Leedham Elvidge at the University of East Anglia in England examined air samples taken on board commercial aircraft cruising at the lower edge of the stratosphere. She found high levels of DCM, especially over the Indian subcontinent and Southeast Asia, and particularly during the Asian monsoon season, when strong updrafts fast-track air from the ground to the stratosphere. It seems they were taking DCM along for the ride.
How much should we worry? Ryan Hossaini, an atmospheric chemist at Lancaster University, recently did the math. He calculated that DCM currently contributes less than 10% of the chlorine in the ozone layer. But on current emission trends, it could be that could delay the ozone hole’s recovery by 30 years, until at least 2095, he suggested.
Others share that concern. “Growing quantities of DCM are leaking into the stratosphere, where it is exceptionally effective in destroying the ozone,” says David Rowley, an atmospheric chemist at the University College London, who was not involved in the research. “The potential for DCM to affect the global ozone budget is profound.”
Other culprits identified
Alarm bells are ringing about dozens of other short-lived, potentially ozone-destroying chlorine compounds accumulating in the atmosphere as a result of fast-rising global manufacturing. They include 1,2-dichloroethane, a chemical widely used in the manufacture of PVC pipes. There are few atmospheric measurements of this compound yet, “but sporadic data suggest it is a significant source of chlorine in the atmosphere,” says Hossaini.
Satellite imagery depicting the annual maximum extent of the ozone hole over Antarctica from 1979 to 2013 (Credit: NASA Goddard Space Flight Center)
The risks of such chemicals reaching the ozone layer are greatest in the tropics, where manufacturing is booming in fast-industrializing countries such as China and India, and where, as luck would have it, atmospheric circulation patterns are favorable. The Asian monsoon can propel the gases to the stratosphere in as little as 10 days, according to unpublished research seen by Yale Environment 360.
Thirty years on, the Montreal Protocol has not begun to come to grips with these chemicals, warns Rowley. “The naïve view until recently,” he says, “was that short-lived [chemicals] didn’t present a threat to stratospheric ozone. Wrong.”
Other loopholes in the protocol are concerning researchers as well. In 2014, colleagues of Leedham Elvidge’s at the University of East Anglia warned that three CFCs supposedly banned under the protocol were turning up in increasing amounts in the clean air blowing round the Southern Ocean and captured at Cape Grim in Tasmania. Johannes Laube, an atmospheric chemist at the University of East Anglia, calculated that global emissions of CFC-113a, once an important feedstock in manufacturing both refrigerants and pyrethroid pesticides, doubled in two years.
How come? It turns out that the Montreal Protocol never completely banned CFCs. “CFC-113a is covered by a loophole that allows industries to apply for exemptions,” Laube says. Confidentiality clauses in the treaty about these exemptions mean that “we simply don’t know if we have found exempted emissions, or if they are from some illegal manufacture somewhere. Either way, they are increasing fast, which makes this worrying.” Trade in banned ozone-depleting chemicals has declined in the past decade, but remains a problem, and has been documented particularly for hydrochlorofluorocarbons.
Science divided on recovery
Scientists knew recovery of the ozone layer would take time because of the long lifetimes of many of the dangerous compounds we unleashed in past decades. But last year, Susan Solomon of MIT — who back in the 1980s became one of the world’s most celebrated scientists for uncovering the chemistry of the polar stratospheric clouds — declared that she had detected the first “fingerprints” of the hole closing. “The onset of healing of Antarctic ozone loss has now emerged,” she wrote.
But other researchers remain cautious. There have been some recent bumper springtime holes in Antarctic ozone. The 2015 hole was the fourth largest since 1991, peaking at an area larger than the continent of North America. It was also deeper than other recent holes and lasted longer. 2016 was also worse than average and 2017 is expected to be severe, too.
Solomon blamed 2015 on the Calbuco volcano in Chile, which ejected sulphur particles that enhanced the ozone-destroying properties of polar stratospheric clouds. But Susan Strahan of NASA’s Goddard Space Flight Center warns that the size of the hole in any given year is still dominated by year-to-year variations in the temperature of the stratosphere and the vagaries of meteorology. “The signature of ozone recovery is not quite there yet,” she says, adding that day will come, but we may have to wait until the 2030s.
Meanwhile at the other end of the planet, ozone losses over the Arctic may still be worsening. The Arctic is less susceptible to the formation of ozone holes than Antarctica, because the weather is messier. The stable air that causes the ultra-cold conditions where polar stratospheric clouds form in Antarctica is much less likely. But it does happen whenever temperatures get cold enough for polar stratospheric clouds to form.
A deep hole briefly formed over the Arctic in 2011. In places, more than 80 percent of the ozone was destroyed, twice the loss in the worst previous years, 1996 and 2005. In both the past two winters, researchers saw polar stratospheric clouds over parts of Britain, says Jonathan Shanklin of the British Antarctic Survey. But they were brief and did not lead to major ozone loss.
Global warming a contributing factor
Shanklin says an important reason for the sluggish recovery of the ozone layer is global warming. As increased levels of greenhouse gases such as carbon dioxide trap more solar heat radiating from the Earth’s surface, less warmth reaches the stratosphere, which cools as a result. This trend has been evident for almost 40 years. A colder stratosphere improves conditions for ozone loss. Climate change “could delay the recovery of the ozone hole well into the second half of this century,” he says.
Should we be frightened? Some of the crazier hype in the early days of the ozone hole — like blind sheep in Patagonia and collapsing marine ecosystems — proved to be nonsense. But the raised risk of skin cancers from the extra ultraviolet radiation streaming through the thinned ozone layer is real enough — particularly for reckless white-skinned sunbathers. The ozone layer is still as thin as it was 30 years ago.
The good news is that without the Montreal Protocol things would have been a great deal worse, says Martyn Chipperfield, an atmospheric chemist at the University of Leeds. The Antarctic hole would be 40% bigger than it is; the ozone layer over Europe and North America would be 10% thinner; the 2011 Arctic hole would have been Antarctic-sized; and we would be looking at about two million more cases of skin cancers by 2030, according to research conducted by Chipperfield and colleagues.
Even so, the idea that the Montreal Protocol is doing its job and the recovery is under way begins to look complacent. If emissions of uncontrolled ozone-depleting chemicals such as DCM continue rising, then the gains could be lost. The answer is obvious. “We should be looking into controlling DCM and other solvents, much in the same way as we did CFCs,” says Leedham Elvidge.
The World Meteorological Organization and other UN agencies overseeing the protocol acknowledge that DCM and other short-lived ozone depleting substances “are an emerging issue for stratospheric ozone,” but the government signatories have yet to take action to limit their emissions.
That would involve getting rid of a far wider range of chemicals than so far done under the protocol. Protecting the ozone layer “presents a much greater industrial and political challenge than previously thought,” says Rowley. Thirty years on, there is evidently still a lot to do.
Fred Pearce is a freelance author and journalist based in the UK, and a contributing writer for Yale Environment 360, where this post originally appeared.
Weekly Newsletter
Get building science and energy efficiency advice, plus special offers, in your inbox.













0 Comments
Log in or create an account to post a comment.
Sign up Log in