
Look at that photo above. Is condensation really responsible for all that moisture you see? We use the term “condensation” a lot when talking about water vapor interacting with materials. But when it comes to porous materials like wood, concrete, and drywall, that’s not really what’s happening. Ready to explore this rabbit hole?
Condensation occurs with nonporous materials
There is some condensation in that photo above. It’s the water dripping off that PVC pipe. Condensation is what happens when a nonporous material drops below the dew point temperature of the air it’s in contact with.

On what kind of materials does condensation occur? Here are a few:
- glass shower door
- mirror (also glass)
- metal window frame
- plastic pipes
- foil jacket on ducts
- porous materials with nonporous coatings like wood with varnish
In cases of true condensation, the water vapor interacts only with the outer layer of the material. And the interaction depends on the temperature of that material. When the material is above the dew point temperature, the surface is dry. When the material drops below the dew point, water vapor molecules stop just bouncing off and start sticking.
Adsorption occurs in porous materials
In the lead photo above, the wood is saturated with water. It started getting wet even before the temperature of the wood dropped below the dew point temperature. Why? Because the water vapor in the air is not just outside the material. It’s also inside the material, floating through the pores and sticking to the pore walls. The relevant psychrometric quantity in this case is relative humidity, not dew point temperature.
![Sorption isotherms for three porous materials [Courtesy of Prof. Chris Timusk]](https://www.energyvanguard.com/wp-content/uploads/2023/09/sorption-isotherm-porous-materials-chris-timusk-thesis.png)
After a bit, each curve flattens out as the pore surfaces get their initial coverage with water molecules. And then the moisture content of each porous material begins to rise steeply when the relative humidity gets high. That’s when the pores start becoming full of water.
Adsorption vs. absorption
When I wrote the word “adsorption,” that wasn’t a typo. Adsorption (the subject of my doctoral dissertation) is when molecules stick to the surface. In the case of water vapor and porous materials, the water molecules stick to the pore walls, one layer at a time. Those layers have a special name, too: monolayers.
Eventually, though, the pores fill up. Then you have liquid water and the material is now absorbing water.
In my freshman chemistry class, I learned a great way to distinguish between adsorption and absorption. There was a cartoon in my textbook that illustrated this concept with a pie. When someone throws a pie in your face, you’ve adsorbed it. When you eat a pie, you’ve absorbed it.
Understanding sorption isotherms
Now, back to the sorption isotherms. That steep rise at high relative humidity doesn’t depend on the dew point temperature. Instead, it depends on how big the pores are, the shape and distribution of the pores, and the relative humidity. This rabbit hole is a deep one, but if you want to understand things like buckling floors, wet sheathing, and mold, you may want to jump in.

As you can see in the sorption isotherm graph above, each material has its own shape when you plot this graph. The way building scientists create sorption isotherm graphs is with carefully controlled experiments with equipment like you see above.
They put a sample in the chamber. It sits on a scale that measures the mass. They gradually increase the relative humidity inside the chamber while maintaining a constant temperature (iso = same, therm = temperature). The mass increases as the relative humidity increases. Then they plot the data.
Temperature variation
Now let’s talk about temperature. Each sorption isotherm is measured at a constant temperature. But what happens when we measure sorption isotherms for the same material at different temperatures? It’s what we expect would happen, right? As we lower the temperature, the material absorbs more water.
![Moisture content of wood at different temperatures and relative humidity [Source: Forest Products Laboratory, Research Note FPL-RN-0268]](https://www.energyvanguard.com/wp-content/uploads/2023/09/moisture-content-wood-vs-relative-humidity.jpg)
Down the rabbit hole
I discussed this topic in my book, but let’s take a quick look here, too. The water molecule, one oxygen and two hydrogens, is polar. The oxygen lacks two electrons from having a complete outer shell. The hydrogen has one electron in its outer shell and would like one more. So the oxygen shares one electron with each hydrogen by creating covalent bonds. That makes the oxygen side slightly negative, the hydrogen side slightly positive.
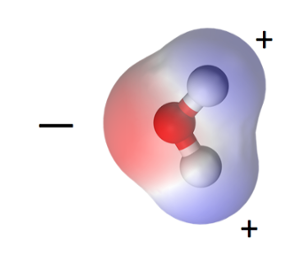
This polarity has enormous ramifications for how water interacts with materials. In fact, it has enormous implications for life on Earth. It’s this polarity that affects how it interacts with materials. That applies to what’s going on inside the pores of porous materials and on the surface of nonporous materials.
Want to go deeper? See chapter 6 of my book. Also, these two articles by Dr. Joseph Lstiburek can open up whole new pores in this rabbit hole:
It’s All Relative
Wood Is Good…But Strange
But the big takeaway here is that when we’re talking about porous materials, it’s not really condensation that’s happening. The term I like to use is moisture accumulation. And now that you’ve got this under your belt, you’re ready to join the building science nerds who argue about whether or not you can get condensation on a sponge.
____________________________________________________________________
Allison A. Bailes III, PhD is a speaker, writer, building science consultant, and the founder of Energy Vanguard in Decatur, Georgia. He has a doctorate in physics and is the author of a bestselling book on building science. He also writes the Energy Vanguard Blog. For more updates, you can subscribe to the Energy Vanguard newsletter and follow him on LinkedIn. Images courtesy of author, except where noted.
Weekly Newsletter
Get building science and energy efficiency advice, plus special offers, in your inbox.














8 Comments
CYNICS ARE US!
I would enjoy seeing more articles like this on this site. One thing I am not seeing is
any data on the change in R-Value which undoubtedly occurs as the moisture
content of wood (or other materials such as cellulose, fiberglass, concrete, etc.)
increases. I strongly suspect that most "fuzzy insulations" have a large drop in R-Value
associated with absorption. And, that those that are capable of absorption are organic
(such as cellulose, wool, hemp, etc.). I also suspect that non-organic insulations (such
as fiberglass, mineral wool, etc.) may also show such a drop due to moisture
accumulation on the surfaces of the fibers from adsorption (lets face it all those miles
of fibers have a lot of surface area). Unfortunately, the organic based insulations
probably lose R-Value due to both ( I'm ignoring the foams here ). Is It Possible
that the insulation industries find it economically inconvenient to test and report
on the change in R-Value in their product due to increasing moisture content.
Maybe I'm just excessively cynical ! But, it's long past time the people advocating
cellulose in walls because of its so-called buffering abilities start thinking about
this - OH Wait! they can't think about it! There isn't any data! Yep, I'm just overly
cynical!
Then there's the drying time! How long does it take for say 5.5 inches of any given
insulation to dry relative to the other type of insulation? Are the organics noticeably
slower than non-organics? How about slumping/settling with increasing weight due
to Adsorption/Absorption? We really need a lot of engineering studies on these
sorts of things but who's going to pay for them? Not the insulation industry, I assure you!
Allison,
I wish you wouldn't write stuff like this. I find it fascinating, but when I describe it to my wife her eyes glaze over. That's fine now - she can put it down to me being a harmless eccentric, but in few years it may be just enough for her to get me put in a home.
About six months ago I made a post on this subject in the Q&A: https://www.greenbuildingadvisor.com/question/wood-air-and-water-the-interplay-of-temperature-humidity-and-wood-moisture-content
I thought it was really something meaningful, but it didn't get the reaction I expected.
So let me try to recast both my post and the article: The issue isn't so much that all moisture isn't condensation. It's that moisture levels below the point of condensation can be problematic for wood. In particular, in cool temperatures "dry" wood can have moisture contents high enough to support the growth of mold and rot.
DC,
Very good point - as were the ones you made in your post.
Great article… should have studied Material Science instead of Chemical Engneering.
Sir, I don't wish to offend but I respectfully question this. I'm not even a contractor but I must say that, to me, it seems counter-intuitive to accept that moisture is not condensing on that wood and that, therefore, it's from ambient moisture that has accumulated in the wood.
If the wood is cold enough and exposed to enough ambient moisture, it does condense on the wood. After all, we do see condensation on the undersides of cold roof sheathing so we know that wood can be a condensing surface. I suspect that there is thermal-bridging happening with the piece in question and it gets cold enough that the ambient moisture cannot absorb quickly enough so it condenses.
The wood in the lead photo is said to be "saturated with water." If the board is truly saturated to the extent that it is dripping so much, how long would that take to completely dry out? My understanding is that, yes wood gets wet in a house under certain conditions but we rely on the knowledge or assumption, at least, that it then dries out completely and all is well if that is unimpeded. Maybe I overestimate how long it'd take for that piece to dry out sufficiently. I know an non-pressure-treated 2x4 can stay afloat on water a long time before sinking and I assume it would need to be at the point of sinking before it would drip that much.
In my mind, if wood becomes saturated like that, it would suggest that the house may be in danger of eventual collapse from rot and mold directly related to such effected pieces of wood. If this is happening on that piece, then it must also be happening on other similarly situated thermal bridges nearby it.
Nevertheless, I will remain open-minded.
Handyhomehacker,
These articles help explain the difference between condensation and adsorbtion:
https://buildingscience.com/documents/building-science-insights-newsletters/bsi-099-its-all-relative
https://buildingscience.com/documents/digests/bsd-138-moisture-and-materials
Handyhomehacker is not completely wrong, and I also question Allison's use of that photo and its explanation. As Allison says in the article, adsorption occurs on wood until the surfaces of the pores can't hold any more, and then absorption continues to fill the rest of the empty spaces. Dr. Joe refers to this behavior as "capillary suction" or "capillary condensation." We also see from the adsorption isotherms that, by the nature of the testing, the test material is never chilled below the dewpoint of the surrounding air. Of course this is the case because we're testing adsorption, not absorption or condensation. But what happens when the test item (wood) is chilled to below the dewpoint of the air around it? Dry wood will start adsorbing the moisture, then absorbing the moisture. Eventually (at 100% RH in the pores), the pores reach their maximum saturation and they simply can't hold any more moisture. But the wood is still chilled below the dewpoint, so moisture from the air now condenses on the surface of the wood, just like a non-porous material. I'd suggest that once moisture is dripping from the wood (as in the lead photo), it is in condensation mode rather than ad/absorption mode.
I guess this all gives me a reason to go back and read chapter 6 of Allison's book.
Log in or create an account to post a comment.
Sign up Log in